Cannabinoids in Pain Management and Palliative Medicine
- PMID: 29017688
- PMCID: PMC5645627
- DOI: 10.3238/arztebl.2017.0627
Cannabinoids in Pain Management and Palliative Medicine
Abstract
Background: There are conflicting interpretations of the evidence regarding the efficacy, tolerability, and safety of cannabinoids in pain management and palliative medicine.
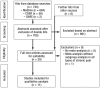
Methods: We conducted a systematic review (SR) of systematic reviews of randomized controlled trials (RCT) and prospective long-term observational studies of the use of cannabinoids in pain management and palliative medicine. Pertinent publications from January 2009 to January 2017 were retrieved by a selective search in the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, and Medline. The methodological quality of the SRs was assessed with the AMSTAR instrument, and the clinical relevance of quantitative data syntheses was assessed according to the standards of the Cochrane Collaboration.
Results: Of the 750 publications identified, 11 SRs met the inclusion criteria; 3 of them were of high and 8 of moderate methodological quality. 2 prospective long-term observational studies with medical cannabis and 1 with tetrahydrocannabinol/cannabidiol spray (THC/CBD spray) were also analyzed. There is limited evidence for a benefit of THC/CBD spray in the treatment of neuropathic pain. There is inadequate evidence for any benefit of cannabinoids (dronabinol, nabilone, medical cannabis, or THC/CBD spray) to treat cancer pain, pain of rheumatic or gastrointestinal origin, or anorexia in cancer or AIDS. Treatment with cannabis-based medicines is associated with central nervous and psychiatric side effects.
Conclusion: The public perception of the efficacy, tolerability, and safety of cannabis-based medicines in pain management and palliative medicine conflicts with the findings of systematic reviews and prospective observational studies conducted according to the standards of evidence-based medicine.
Figures
Comment in
-
Considerable Heterogeneity.Dtsch Arztebl Int. 2018 Mar 2;115(9):143. doi: 10.3238/arztebl.2018.0143a. Dtsch Arztebl Int. 2018. PMID: 29563008 Free PMC article. No abstract available.
References
-
- Bundesanzeiger. Gesetz zur Änderung betäubungsmittelrechtlicher und anderer Vorschriften. Bundesgesetzblatt. 2017;1:403–406.
-
- Müller-Vahl K, Grotenhermen F. Medizinisches Cannabis: Die wichtigsten Änderungen. Dtsch Arztebl. 2017;114:A 352–A 356.
-
- Baethge C. Evidenzbasierte Medizin. In der Versorgung angekommen, aber noch nicht heimisch. Dtsch Arztebl. 2014;111:A 1636–A 1640. - PubMed
-
- Häuser W, Klose P, Welsch P, Petzke F, Nothacker M, Kopp I. [Methodology of the development of the updated LONTS guidelines for long-term administration of opioids in noncancer pain] Schmerz. 2015;29:8–34. - PubMed
-
- Andrew Moore R, Eccleston C, Derry S, et al. „Evidence“ in chronic pain—establishing best practice in the reporting of systematic reviews. Pain. 2010;15:386–389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous