Hypothermic oxygenated machine perfusion (HOPE) for orthotopic liver transplantation of human liver allografts from extended criteria donors (ECD) in donation after brain death (DBD): a prospective multicentre randomised controlled trial (HOPE ECD-DBD)
- PMID: 29018070
- PMCID: PMC5652559
- DOI: 10.1136/bmjopen-2017-017558
Hypothermic oxygenated machine perfusion (HOPE) for orthotopic liver transplantation of human liver allografts from extended criteria donors (ECD) in donation after brain death (DBD): a prospective multicentre randomised controlled trial (HOPE ECD-DBD)
Abstract
Introduction: Orthotopic liver transplantation (OLT) has emerged as the mainstay of treatment for end-stage liver disease. In an attempt to improve the availability of donor allografts and reduce waiting list mortality, graft acceptance criteria were extended increasingly over the decades. The use of extended criteria donor (ECD) allografts is associated with a higher incidence of primary graft non-function and/or delayed graft function. As such, several strategies have been developed aiming at reconditioning poor quality ECD liver allografts. Hypothermic oxygenated machine perfusion (HOPE) has been successfully tested in preclinical experiments and in few clinical series of donation after cardiac death OLT.
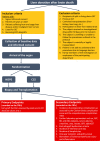
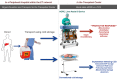
Methods and analysis: HOPE ECD-DBD is an investigator-initiated, open-label, phase-II, prospective multicentre randomised controlled trial on the effects of HOPE on ECD allografts in donation after brain death (DBD) OLT. Human whole organ liver grafts will be submitted to 1-2 hours of HOPE (n=23) via the portal vein before implantation and are going to be compared with a control group (n=23) of patients transplanted after conventional cold storage. Primary (peak and Δ peak alanine aminotransferase within 7 days) and secondary (aspartate aminotransferase, bilirubin and international normalised ratio, postoperative complications, early allograft dysfunction, duration of hospital and intensive care unit stay, 1-year patient and graft survival) endpoints will be analysed within a 12-month follow-up. Extent of ischaemia-reperfusion (I/R) injury will be assessed using liver tissue, perfusate, bile and serum samples taken during the perioperative phase of OLT.
Ethics and dissemination: The study was approved by the institutional review board of the RWTH Aachen University, Aachen, Germany (EK 049/17). The current paper represent the pre-results phase. First results are expected in 2018.
Trial registration number: NCT03124641.
Keywords: donation after brain death; extended criteria donor; hypothermic oxygenated machine perfusion.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
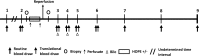
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
