Seasonal influenza vaccine effectiveness against laboratory-confirmed influenza in 2015-2016: a hospital-based test-negative case - control study in Lithuania
- PMID: 29018073
- PMCID: PMC5652622
- DOI: 10.1136/bmjopen-2017-017835
Seasonal influenza vaccine effectiveness against laboratory-confirmed influenza in 2015-2016: a hospital-based test-negative case - control study in Lithuania
Abstract
Objective: A case-control study was conducted to assess seasonal influenza vaccine effectiveness (SIVE) during the 2015-2016 influenza season.
Methods: A study was performed in three departments in Lithuania between 1 December 2015 and 1 May 2016. Data on demographic and clinical characteristics including influenza vaccination status were collected from the patients recommended to receive the seasonal influenza vaccine. Influenza virus infection was confirmed by multiplex reverse transcription polymerase chain reaction (RT-PCR) .
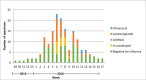
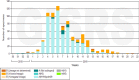
Results: Ninety-one (56.4%) of the 163 included subjects were ≥65 years old. Fifteen (9.2%) subjects were vaccinated against influenza at least 2 weeks before the onset of influenza symptoms, 12 of them were ≥65 years old. Of the 72 (44.2%) influenza virus positive cases, 65 (39.9%) were confirmed with influenza A (including 50 cases of influenza A(H1N1)pdm09), eight (4.9%) were confirmed with influenza B and one was a co-infection. Unadjusted SIVE against any influenza, influenza type A and influenza A(H1N1)pdm09 was 57% (95% CI -41% to 87%), 52% (95% CI -57% to 85%) and 70% (95% CI -43% to 94%) respectively.
Conclusion: Although SIVE estimates were not statistically significant the point estimates suggest moderate effectiveness against influenza type A.
Keywords: effectiveness; influenza; influenza vaccination; lithuania; test-negative case-control study.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Figures
References
-
- Commission of the European Communities. Council recommendation of 13 July 2009 on Seasonal Influenza vaccination. Off J 2009;09:1–12. L 353, 13/7/.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical