Suppression and Contrast Normalization in Motion Processing
- PMID: 29018158
- PMCID: PMC5678028
- DOI: 10.1523/JNEUROSCI.1572-17.2017
Suppression and Contrast Normalization in Motion Processing
Abstract
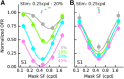
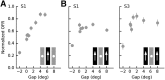
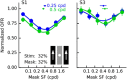
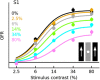
Sensory neurons are activated by a range of stimuli to which they are said to be tuned. Usually, they are also suppressed by another set of stimuli that have little effect when presented in isolation. The interactions between preferred and suppressive stimuli are often quite complex and vary across neurons, even within a single area, making it difficult to infer their collective effect on behavioral responses mediated by activity across populations of neurons. Here, we investigated this issue by measuring, in human subjects (three males), the suppressive effect of static masks on the ocular following responses induced by moving stimuli. We found a wide range of effects, which depend in a nonlinear and nonseparable manner on the spatial frequency, contrast, and spatial location of both stimulus and mask. Under some conditions, the presence of the mask can be seen as scaling the contrast of the driving stimulus. Under other conditions, the effect is more complex, involving also a direct scaling of the behavioral response. All of this complexity at the behavioral level can be captured by a simple model in which stimulus and mask interact nonlinearly at two stages, one monocular and one binocular. The nature of the interactions is compatible with those observed at the level of single neurons in primates, usually broadly described as divisive normalization, without having to invoke any scaling mechanism.SIGNIFICANCE STATEMENT The response of sensory neurons to their preferred stimulus is often modulated by stimuli that are not effective when presented alone. Individual neurons can exhibit multiple modulatory effects, with considerable variability across neurons even in a single area. Such diversity has made it difficult to infer the impact of these modulatory mechanisms on behavioral responses. Here, we report the effects of a stationary mask on the reflexive eye movements induced by a moving stimulus. A model with two stages, each incorporating a divisive modulatory mechanism, reproduces our experimental results and suggests that qualitative variability of masking effects in cortical neurons might arise from differences in the extent to which such effects are inherited from earlier stages.
Keywords: masking; motion; normalization.
Copyright © 2017 the authors 0270-6474/17/3711051-16$15.00/0.
Figures
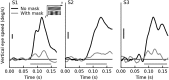
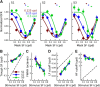
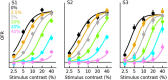
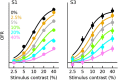
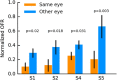

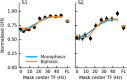
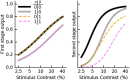

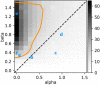
Similar articles
-
Contrast Normalization Accounts for Binocular Interactions in Human Striate and Extra-striate Visual Cortex.J Neurosci. 2020 Mar 25;40(13):2753-2763. doi: 10.1523/JNEUROSCI.2043-19.2020. Epub 2020 Feb 14. J Neurosci. 2020. PMID: 32060172 Free PMC article.
-
Dichoptic visual masking reveals that early binocular neurons exhibit weak interocular suppression: implications for binocular vision and visual awareness.J Cogn Neurosci. 2004 Jul-Aug;16(6):1049-59. doi: 10.1162/0898929041502788. J Cogn Neurosci. 2004. PMID: 15298791
-
A Model of Binocular Motion Integration in MT Neurons.J Neurosci. 2016 Jun 15;36(24):6563-82. doi: 10.1523/JNEUROSCI.3213-15.2016. J Neurosci. 2016. PMID: 27307243 Free PMC article.
-
Characterizing and interpreting the influence of internal variables on sensory activity.Curr Opin Neurobiol. 2017 Oct;46:84-89. doi: 10.1016/j.conb.2017.07.006. Epub 2017 Aug 24. Curr Opin Neurobiol. 2017. PMID: 28841439 Free PMC article. Review.
-
Why context matters? Divisive normalization and canonical microcircuits in psychiatric disorders.Neurosci Res. 2020 Jul;156:130-140. doi: 10.1016/j.neures.2019.10.002. Epub 2019 Oct 16. Neurosci Res. 2020. PMID: 31628970 Review.
Cited by
-
Manipulating the Fourier spectra of stimuli comprising a two-frame kinematogram to study early visual motion-detecting mechanisms: Perception versus short latency ocular-following responses.J Vis. 2023 Sep 1;23(10):11. doi: 10.1167/jov.23.10.11. J Vis. 2023. PMID: 37725387 Free PMC article.
-
Ocular-following responses to broadband visual stimuli of varying motion coherence.J Vis. 2024 Dec 2;24(13):4. doi: 10.1167/jov.24.13.4. J Vis. 2024. PMID: 39630464 Free PMC article.
-
Weighted power summation and contrast normalization mechanisms account for short-latency eye movements to motion and disparity of sine-wave gratings and broadband visual stimuli in humans.J Vis. 2024 Aug 1;24(8):14. doi: 10.1167/jov.24.8.14. J Vis. 2024. PMID: 39186301 Free PMC article.
-
Short-latency ocular-following responses: Weighted nonlinear summation predicts the outcome of a competition between two sine wave gratings moving in opposite directions.J Vis. 2020 Jan 24;20(1):1. doi: 10.1167/jov.20.1.1. J Vis. 2020. PMID: 31995136 Free PMC article.
References
-
- Albrecht DG, Geisler WS, Frazor RA, Crane AM (2002) Visual cortex neurons of monkeys and cats: temporal dynamics of the contrast response function. J Neurophysiol 88:888–913. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
