The human blood parasite Schistosoma mansoni expresses extracellular tegumental calpains that cleave the blood clotting protein fibronectin
- PMID: 29018227
- PMCID: PMC5635006
- DOI: 10.1038/s41598-017-13141-5
The human blood parasite Schistosoma mansoni expresses extracellular tegumental calpains that cleave the blood clotting protein fibronectin
Abstract
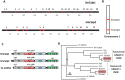
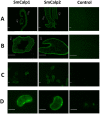
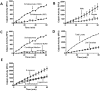
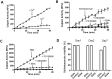
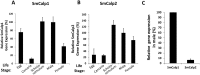
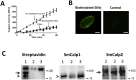
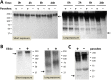
Schistosomes are intravascular, parasitic flatworms that cause debilitating disease afflicting >200 million people. Proteins expressed at the host-parasite interface likely play key roles in modifying the worm's local environment to ensure parasite survival. Proteomic analysis reveals that two proteases belonging to the calpain family (SmCalp1 and SmCalp2) are expressed in the Schistosoma mansoni tegument. We have cloned both; while highly conserved in domain organization they display just 31% amino acid sequence identity. Both display high relative expression in the parasite's intravascular life forms. Immunolocalization and activity based protein profiling experiments confirm the presence of the enzymes at the host-parasite interface. Living parasites exhibit surface calpain activity that is blocked in the absence of calcium and in the presence of calpain inhibitors (E64c, PD 150606 and calpastatin). While calpains are invariably reported to be exclusively intracellular (except in diseased or injured tissues), our data show that schistosomes display unique, constitutive, functional extracellular calpain activity. Furthermore we show that the worms are capable of cleaving the host blood clotting protein fibronectin and that this activity can be inhibited by E64c. We hypothesize that SmCalp1 and/or SmCalp2 perform this cleavage function to impede blood clot formation around the worms in vivo.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The blood fluke Schistosoma mansoni cleaves the coagulation protein high molecular weight kininogen (HK) but does not generate the vasodilator bradykinin.Parasit Vectors. 2018 Mar 14;11(1):182. doi: 10.1186/s13071-018-2704-0. Parasit Vectors. 2018. PMID: 29540224 Free PMC article.
-
Molecular characterization and functional analysis of the Schistosoma mekongi Ca2+-dependent cysteine protease (calpain).Parasit Vectors. 2019 Jul 30;12(1):383. doi: 10.1186/s13071-019-3639-9. Parasit Vectors. 2019. PMID: 31362766 Free PMC article.
-
Intravascular Schistosoma mansoni Cleave the Host Immune and Hemostatic Signaling Molecule Sphingosine-1-Phosphate via Tegumental Alkaline Phosphatase.Front Immunol. 2018 Jul 30;9:1746. doi: 10.3389/fimmu.2018.01746. eCollection 2018. Front Immunol. 2018. PMID: 30105025 Free PMC article.
-
The enigmatic heptalaminate surface membrane of intravascular schistosomes.Trends Parasitol. 2025 Mar;41(3):177-187. doi: 10.1016/j.pt.2025.01.005. Epub 2025 Feb 5. Trends Parasitol. 2025. PMID: 39915200 Review.
-
Schistosome secretomes.Acta Trop. 2022 Dec;236:106676. doi: 10.1016/j.actatropica.2022.106676. Epub 2022 Sep 14. Acta Trop. 2022. PMID: 36113567 Review.
Cited by
-
Convergent evolution of a genotoxic stress response in a parasite-specific p53 homolog.Proc Natl Acad Sci U S A. 2022 Sep 13;119(37):e2205201119. doi: 10.1073/pnas.2205201119. Epub 2022 Sep 6. Proc Natl Acad Sci U S A. 2022. PMID: 36067283 Free PMC article.
-
Sm-p80-based schistosomiasis vaccine: double-blind preclinical trial in baboons demonstrates comprehensive prophylactic and parasite transmission-blocking efficacy.Ann N Y Acad Sci. 2018 Aug;1425(1):38-51. doi: 10.1111/nyas.13942. Ann N Y Acad Sci. 2018. PMID: 30133707 Free PMC article.
-
Hidden in plain sight: How helminths manage to thrive in host blood.Front Parasitol. 2023 Mar 10;2:1128299. doi: 10.3389/fpara.2023.1128299. eCollection 2023. Front Parasitol. 2023. PMID: 39816845 Free PMC article. Review.
-
Eudiplozoon nipponicum (Monogenea, Diplozoidae) and its adaptation to haematophagy as revealed by transcriptome and secretome profiling.BMC Genomics. 2021 Apr 15;22(1):274. doi: 10.1186/s12864-021-07589-z. BMC Genomics. 2021. PMID: 33858339 Free PMC article.
-
Potential biomarker and composite efficacy readout for human clinical trials of schistosomiasis vaccine in Africa.Sci Rep. 2025 Jul 2;15(1):23251. doi: 10.1038/s41598-025-05730-6. Sci Rep. 2025. PMID: 40603376 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

