Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles
- PMID: 29018242
- PMCID: PMC5635018
- DOI: 10.1038/s41598-017-12926-y
Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles
Abstract
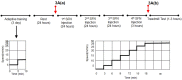
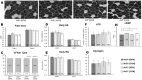
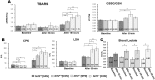
Sulforaphane (SFN) plays an important role in preventing oxidative stress by activating the nuclear factor (erythroid derived 2)-like 2 (Nrf2) signalling pathway. SFN may improve exercise endurance capacity by counteracting oxidative stress-induced damage during exercise. We assessed running ability based on an exhaustive treadmill test (progressive-continuous all-out) and examined the expression of markers for oxidative stress and muscle damage. Twelve- to 13-week-old Male wild-type mice (Nrf2 +/+) and Nrf2-null mice (Nrf2 -/-) on C57BL/6J background were intraperitoneally injected with SFN or vehicle prior to the test. The running distance of SFN-injected Nrf2 +/+ mice was significantly greater compared with that of uninjected mice. Enhanced running capacity was accompanied by upregulation of Nrf2 signalling and downstream genes. Marker of oxidative stress in SFN-injected Nrf2 +/+ mice were lower than those in uninjected mice following the test. SFN produced greater protection against muscle damage during exhaustive exercise conditions in Nrf2 +/+ mice than in Nrf2 -/- mice. SFN-induced Nrf2 upregulation, and its antioxidative effects, might play critical roles in attenuating muscle fatigue via reduction of oxidative stress caused by exhaustive exercise. This in turn leads to enhanced exercise endurance capacity. These results provide new insights into SFN-induced upregulation of Nrf2 and its role in improving exercise performance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Acute exercise stress promotes Ref1/Nrf2 signalling and increases mitochondrial antioxidant activity in skeletal muscle.Exp Physiol. 2016 Mar;101(3):410-20. doi: 10.1113/EP085493. Epub 2016 Jan 23. Exp Physiol. 2016. PMID: 26682532
-
Hypoxia preconditioning promotes endurance exercise capacity of mice by activating skeletal muscle Nrf2.J Appl Physiol (1985). 2019 Nov 1;127(5):1267-1277. doi: 10.1152/japplphysiol.00347.2019. Epub 2019 Sep 5. J Appl Physiol (1985). 2019. PMID: 31487225
-
Nrf2 Activation Enhances Muscular MCT1 Expression and Hypoxic Exercise Capacity.Med Sci Sports Exerc. 2020 Aug;52(8):1719-1728. doi: 10.1249/MSS.0000000000002312. Med Sci Sports Exerc. 2020. PMID: 32079911
-
The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical.Antioxidants (Basel). 2020 Jun 13;9(6):521. doi: 10.3390/antiox9060521. Antioxidants (Basel). 2020. PMID: 32545803 Free PMC article. Review.
-
Modulation of mitochondrial functions by the indirect antioxidant sulforaphane: a seemingly contradictory dual role and an integrative hypothesis.Free Radic Biol Med. 2013 Dec;65:1078-1089. doi: 10.1016/j.freeradbiomed.2013.08.182. Epub 2013 Aug 30. Free Radic Biol Med. 2013. PMID: 23999506 Review.
Cited by
-
Effects of different-intensity exercise and creatine supplementation on mitochondrial biogenesis and redox status in mice.Iran J Basic Med Sci. 2022 Aug;25(8):1009-1015. doi: 10.22038/IJBMS.2022.65047.14321. Iran J Basic Med Sci. 2022. PMID: 36159328 Free PMC article.
-
Novel treatment strategies for chronic kidney disease: insights from the animal kingdom.Nat Rev Nephrol. 2018 Apr;14(4):265-284. doi: 10.1038/nrneph.2017.169. Epub 2018 Jan 15. Nat Rev Nephrol. 2018. PMID: 29332935 Review.
-
The Effects of Aerobic-Resistance Training and Broccoli Supplementation on Plasma Dectin-1 and Insulin Resistance in Males with Type 2 Diabetes.Nutrients. 2021 Sep 9;13(9):3144. doi: 10.3390/nu13093144. Nutrients. 2021. PMID: 34579020 Free PMC article. Clinical Trial.
-
Effects of Sulforaphane Treatment on Skeletal Muscle from Exhaustive Exercise-Induced Inflammation and Oxidative Stress Through the Nrf2/HO-1 Signaling Pathway.Antioxidants (Basel). 2025 Feb 12;14(2):210. doi: 10.3390/antiox14020210. Antioxidants (Basel). 2025. PMID: 40002396 Free PMC article.
-
Role for Plant-Derived Antioxidants in Attenuating Cancer Cachexia.Antioxidants (Basel). 2022 Jan 18;11(2):183. doi: 10.3390/antiox11020183. Antioxidants (Basel). 2022. PMID: 35204066 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

