6E11, a highly selective inhibitor of Receptor-Interacting Protein Kinase 1, protects cells against cold hypoxia-reoxygenation injury
- PMID: 29018243
- PMCID: PMC5635128
- DOI: 10.1038/s41598-017-12788-4
6E11, a highly selective inhibitor of Receptor-Interacting Protein Kinase 1, protects cells against cold hypoxia-reoxygenation injury
Abstract
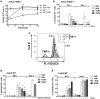
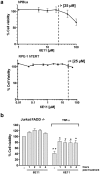
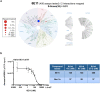
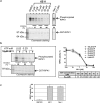
Necroptosis is a programmed cell death pathway that has been shown to be of central pathophysiological relevance in multiple disorders (hepatitis, brain and cardiac ischemia, pancreatitis, viral infection and inflammatory diseases). Necroptosis is driven by two serine threonine kinases, RIPK1 (Receptor Interacting Protein Kinase 1) and RIPK3, and a pseudo-kinase MLKL (Mixed Lineage Kinase domain-Like) associated in a multi-protein complex called necrosome. In order to find new inhibitors for use in human therapy, a chemical library containing highly diverse chemical structures was screened using a cell-based assay. The compound 6E11, a natural product derivative, was characterized as a positive hit. Interestingly, this flavanone compound: inhibits necroptosis induced by death receptors ligands TNF-α (Tumor Necrosis Factor) or TRAIL (TNF-Related Apoptosis-Inducing Ligand); is an extremely selective inhibitor, among kinases, of human RIPK1 enzymatic activity with a nM Kd; has a non-ATP competitive mode of action and a novel putative binding site; is weakly cytotoxic towards human primary blood leukocytes or retinal pigment epithelial cells at effective concentrations; protects human aortic endothelial cells (HAEC) from cold hypoxia/reoxygenation injury more effectively than necrostatin-1 (Nec-1) and Nec-1s. Altogether, these data demonstrate that 6E11 is a novel potent small molecular inhibitor of RIPK1-driven necroptosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

