Systematic Moiety Variations of Ultrashort Peptides Produce Profound Effects on Self-Assembly, Nanostructure Formation, Hydrogelation, and Phase Transition
- PMID: 29018249
- PMCID: PMC5635115
- DOI: 10.1038/s41598-017-12694-9
Systematic Moiety Variations of Ultrashort Peptides Produce Profound Effects on Self-Assembly, Nanostructure Formation, Hydrogelation, and Phase Transition
Abstract
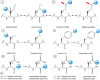
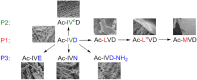
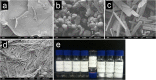
Self-assembly of small biomolecules is a prevalent phenomenon that is increasingly being recognised to hold the key to building complex structures from simple monomeric units. Small peptides, in particular ultrashort peptides containing up to seven amino acids, for which our laboratory has found many biomedical applications, exhibit immense potential in this regard. For next-generation applications, more intricate control is required over the self-assembly processes. We seek to find out how subtle moiety variation of peptides can affect self-assembly and nanostructure formation. To this end, we have selected a library of 54 tripeptides, derived from systematic moiety variations from seven tripeptides. Our study reveals that subtle structural changes in the tripeptides can exert profound effects on self-assembly, nanostructure formation, hydrogelation, and even phase transition of peptide nanostructures. By comparing the X-ray crystal structures of two tripeptides, acetylated leucine-leucine-glutamic acid (Ac-LLE) and acetylated tyrosine-leucine-aspartic acid (Ac-YLD), we obtained valuable insights into the structural factors that can influence the formation of supramolecular peptide structures. We believe that our results have major implications on the understanding of the factors that affect peptide self-assembly. In addition, our findings can potentially assist current computational efforts to predict and design self-assembling peptide systems for diverse biomedical applications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Self-assembly of amphiphilic tripeptides with sequence-dependent nanostructure.Biomater Sci. 2017 Jul 25;5(8):1526-1530. doi: 10.1039/c7bm00304h. Biomater Sci. 2017. PMID: 28518205
-
De novo design and experimental characterization of ultrashort self-associating peptides.PLoS Comput Biol. 2014 Jul 10;10(7):e1003718. doi: 10.1371/journal.pcbi.1003718. eCollection 2014 Jul. PLoS Comput Biol. 2014. PMID: 25010703 Free PMC article.
-
Battacin-Inspired Ultrashort Peptides: Nanostructure Analysis and Antimicrobial Activity.Biomacromolecules. 2019 Jul 8;20(7):2515-2529. doi: 10.1021/acs.biomac.9b00291. Epub 2019 Jun 13. Biomacromolecules. 2019. PMID: 31145611
-
Hierarchical Self-Assembly of Short Peptides: Nanostructure Formation, Function Tailoring, and Applications.Macromol Biosci. 2025 May;25(5):e2400523. doi: 10.1002/mabi.202400523. Epub 2025 Jan 31. Macromol Biosci. 2025. PMID: 39887542 Review.
-
Self-Assembly in Peptides Containing β-and γ-amino Acids.Curr Protein Pept Sci. 2020;21(6):584-597. doi: 10.2174/1389203721666200127112244. Curr Protein Pept Sci. 2020. PMID: 31985376 Review.
Cited by
-
Stimuli-Responsive Membrane Anchor Peptide Nanofoils for Tunable Membrane Association and Lipid Bilayer Fusion.ACS Appl Mater Interfaces. 2022 Dec 21;14(50):55320-55331. doi: 10.1021/acsami.2c11946. Epub 2022 Dec 6. ACS Appl Mater Interfaces. 2022. PMID: 36473125 Free PMC article.
-
Gelation behavior of short protected peptides in organic medium.Soft Matter. 2025 Jun 11;21(23):4751-4760. doi: 10.1039/d5sm00275c. Soft Matter. 2025. PMID: 40407806 Free PMC article.
-
Fabrication of lumen-forming colorectal cancer organoids using a newly designed laminin-derived bioink.Int J Bioprint. 2022 Nov 4;9(1):633. doi: 10.18063/ijb.v9i1.633. eCollection 2023. Int J Bioprint. 2022. PMID: 36866082 Free PMC article.
-
Peptide-Peptide Co-Assembly: A Design Strategy for Functional Detection of C-peptide, A Biomarker of Diabetic Neuropathy.Int J Mol Sci. 2020 Dec 18;21(24):9671. doi: 10.3390/ijms21249671. Int J Mol Sci. 2020. PMID: 33352955 Free PMC article.
-
Synthesis, Antitumor and Antibacterial Studies of New Shortened Analogues of (KLAKLAK)2-NH2 and Their Conjugates Containing Unnatural Amino Acids.Molecules. 2021 Feb 8;26(4):898. doi: 10.3390/molecules26040898. Molecules. 2021. PMID: 33567789 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

