Xiao-Yao-San, a Chinese Medicine Formula, Ameliorates Chronic Unpredictable Mild Stress Induced Polycystic Ovary in Rat
- PMID: 29018356
- PMCID: PMC5614964
- DOI: 10.3389/fphys.2017.00729
Xiao-Yao-San, a Chinese Medicine Formula, Ameliorates Chronic Unpredictable Mild Stress Induced Polycystic Ovary in Rat
Abstract
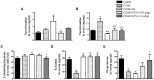
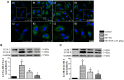
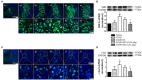
Chronic stress induces endocrine disturbance, which contributes to the development of polycystic ovary syndrome (PCOS), a condition that remains a challenge for clinicians to cope with. The present study investigated the effect of Xiao-Yao-San (XYS), a traditional Chinese medicine formula used for treatment of gynecological disease, on the chronic stress-induced polycystic ovary and its underlying mechanism. Female Sprague-Dwaley rats underwent a 3 weeks chronic unpredictable mild stress (CUMS) procedure to establish the PCOS model, followed by 4 weeks treatment with XYS (0.505 g/kg or 1.01 g/kg) by gavage. Granulosa cells were exposed to noradrenaline (1 mM) in vitro for 24 h, followed by incubation with or without XYS-treated rat serum for 24 h. Post-treatment with XYS ameliorated CUMS-induced irregular estrous cycles and follicles development abnormalities, decrease of estradiol and progesterone level as well as increase of luteinizing hormone in serum, reduced cystic follicles formation and the apoptosis and autophagy of granulosa cells, attenuated the increase in dopamine beta hydroxylase and c-fos level in locus coeruleus, the noradrenaline level in serum and ovarian tissue, and the expression of beta 2 adrenergic receptor in ovarian tissue. Besides, XYS alleviated the reduction of phosphorylation of ribosomal protein S6 kinase polypeptide I and protein kinase B, as well as the increase of microtubule-associated protein light chain 3-I to microtubule-associated protein light chain 3-II conversion both in vivo and in vitro. This study demonstrated XYS as a potential strategy for CUMS induced polycystic ovary, and suggested that the beneficial role of XYS was correlated with the regulation of the sympathetic nerve activity.
Keywords: beta 2 adrenergic receptor; chronic unpredictable stress; granulosa cells autophagy; noradrenaline; polycystic ovary.
Figures







Similar articles
-
Xiao Yao San Improves Depressive-Like Behavior in Rats through Modulation of β-Arrestin 2-Mediated Pathways in Hippocampus.Evid Based Complement Alternat Med. 2014;2014:902516. doi: 10.1155/2014/902516. Epub 2014 Jul 7. Evid Based Complement Alternat Med. 2014. PMID: 25097660 Free PMC article.
-
Xiao-Yao-San Formula Improves Cognitive Ability by Protecting the Hippocampal Neurons in Ovariectomized Rats.Evid Based Complement Alternat Med. 2020 Jun 19;2020:4156145. doi: 10.1155/2020/4156145. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32655660 Free PMC article.
-
Integrating Metabolomics and Network Pharmacology to Explore the Mechanism of Xiao-Yao-San in the Treatment of Inflammatory Response in CUMS Mice.Pharmaceuticals (Basel). 2023 Nov 14;16(11):1607. doi: 10.3390/ph16111607. Pharmaceuticals (Basel). 2023. PMID: 38004472 Free PMC article.
-
Antidepressant effects of the traditional Chinese herbal formula Xiao-Yao-San and its bioactive ingredients.Phytomedicine. 2023 Jan;109:154558. doi: 10.1016/j.phymed.2022.154558. Epub 2022 Nov 21. Phytomedicine. 2023. PMID: 36610123 Review.
-
Efficacy and safety of Chinese herbal medicine Xiao Yao San in hypertension: A systematic review and meta-analysis.Phytomedicine. 2019 Aug;61:152849. doi: 10.1016/j.phymed.2019.152849. Epub 2019 Jan 29. Phytomedicine. 2019. PMID: 31035044
Cited by
-
Effects of TCM on polycystic ovary syndrome and its cellular endocrine mechanism.Front Endocrinol (Lausanne). 2023 May 16;14:956772. doi: 10.3389/fendo.2023.956772. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37260441 Free PMC article. Review.
-
Gyogamdan, a Traditional Medicine Prescription, Ameliorated Dermal Inflammation and Hyperactive Behavior in an Atopic Dermatitis Mouse Model Exposed to Psychological Stress.Evid Based Complement Alternat Med. 2021 Mar 30;2021:6687513. doi: 10.1155/2021/6687513. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 33859711 Free PMC article.
-
Editorial: Traditional Chinese Medicine: Organ Vascular Injury - Volume II.Front Physiol. 2021 Jun 11;12:677858. doi: 10.3389/fphys.2021.677858. eCollection 2021. Front Physiol. 2021. PMID: 34177621 Free PMC article. No abstract available.
-
The role of the autonomic nervous system in polycystic ovary syndrome.Front Endocrinol (Lausanne). 2024 Jan 19;14:1295061. doi: 10.3389/fendo.2023.1295061. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38313837 Free PMC article. Review.
-
Investigating the Role of Autonomic Dysfunction in the Pathogenesis of Depression-Related Dry Eye.Transl Vis Sci Technol. 2025 Jul 1;14(7):4. doi: 10.1167/tvst.14.7.4. Transl Vis Sci Technol. 2025. PMID: 40622202 Free PMC article.
References
-
- Abedini A., Zamberlam G., Lapointe E., Touriqny C., Boyer A., Paquet M., et al. . (2016). WNT5a is required for normal ovarian follicle development and antagonizes gonadotropin responsiveness in granulosa cells by suppressing canonical WNT signaling. FASEB J. 30, 1534–1547. 10.1096/fj.15-280313 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

