IFN-γ-STAT1-iNOS Induces Myeloid Progenitors to Acquire Immunosuppressive Activity
- PMID: 29018448
- PMCID: PMC5614959
- DOI: 10.3389/fimmu.2017.01192
IFN-γ-STAT1-iNOS Induces Myeloid Progenitors to Acquire Immunosuppressive Activity
Abstract
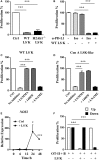
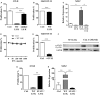
Autoimmune diseases often induce dysregulated hematopoiesis with altered number and function of hematopoietic stem and progenitor cells (HSPCs). However, there are limited studies on the direct regulation of HSPCs on T cells, which are often detrimental to autoimmunity. Here, we found that in a murine model of Concanavalin A-induced autoimmune hepatitis, LSK (Lineage-Sca-1+c-Kit+)-like cells accumulated in liver, spleen, and bone marrow (BM), which were myeloid progenitors (Lineage-Sca-1-c-Kit+) that upregulated Sca-1 expression upon T cell-derived IFN-γ stimulation. Strikingly, BM LSK-like cells from mice induced by Con A to develop autoimmune hepatitis or alternatively myeloid progenitors from wild-type mice possessed strong in vitro suppressive ability. Their suppressive function depended on T cell-derived IFN-γ in a paracrine fashion, which induced STAT1 phosphorylation, inducible nitric oxide synthase expression, and nitric oxide production. Blocking IFN-γ/IFN-γ receptor interaction, knockout of STAT1, or iNOS inhibition abrogated their suppressive function. In addition, the suppressive function was independent of differentiation; mitomycin C-treated myeloid progenitors maintained T cell suppressive ability in vitro. Our data demonstrate a mechanism of inflammation induced suppressive function of myeloid progenitors, which may participate directly in suppressing T cell-mediated immunopathology.
Keywords: IFN-γ; STAT1; T cells; autoimmune disease; bone marrow; immunosuppression; inducible nitride oxide synthase; myeloid progenitors.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

