Overexpression of SlGRAS40 in Tomato Enhances Tolerance to Abiotic Stresses and Influences Auxin and Gibberellin Signaling
- PMID: 29018467
- PMCID: PMC5622987
- DOI: 10.3389/fpls.2017.01659
Overexpression of SlGRAS40 in Tomato Enhances Tolerance to Abiotic Stresses and Influences Auxin and Gibberellin Signaling
Abstract
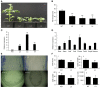
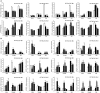
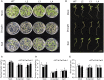
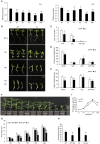
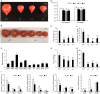
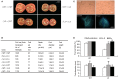
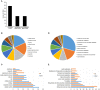
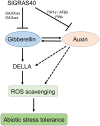
Abiotic stresses are major environmental factors that inhibit plant growth and development impacting crop productivity. GRAS transcription factors play critical and diverse roles in plant development and abiotic stress. In this study, SlGRAS40, a member of the tomato (Solanum lycopersicum) GRAS family, was functionally characterized. In wild-type (WT) tomato, SlGRAS40 was upregulated by abiotic stress induced by treatment with D-mannitol, NaCl, or H2O2. Transgenic tomato plants overexpressing SlGRAS40 (SlGRAS40-OE) were more tolerant of drought and salt stress than WT. SlGRAS40-OE plants displayed pleiotropic phenotypes reminiscent of those resulting from altered auxin and/or gibberellin signaling. A comparison of WT and SlGRAS40-OE transcriptomes showed that the expression of a large number of genes involved in hormone signaling and stress responses were modified. Our study of SlGRAS40 protein provides evidence of how another GRAS plays roles in resisting abiotic stress and regulating auxin and gibberellin signaling during vegetative and reproductive growth in tomato.
Keywords: GRAS transcription factor; SlGRAS40; abiotic stresses; auxin; gibberellin; tomato (Solanum lycopersicum).
Figures


References
-
- Bates L. S., Waldren R. P., Teare I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. 10.1007/BF00018060 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

