Efficacy of individualised diets in patients with irritable bowel syndrome: a randomised controlled trial
- PMID: 29018540
- PMCID: PMC5628288
- DOI: 10.1136/bmjgast-2017-000164
Efficacy of individualised diets in patients with irritable bowel syndrome: a randomised controlled trial
Abstract
Background: Patients with irritable bowel syndrome (IBS) are often placed on diets guided by food intolerance assays, although these have not been validated. We assessed the effects of individualised diets in patients with IBS guided by a leucocyte activation test.
Methods: This is a parallel-group, double-blind, randomised controlled trial of 58 adults with IBS seen at an academic health centre in Northeast USA. Peripheral venous blood was analysed using a leucocyte activation test; individual foods were reported to produce positive or negative results. Participants were randomised to a 4-week diet with either individualised guidance to eliminate foods with positive assay results and allow foods with negative assay results (intervention), or with individualised guidance, matched in rigour and complexity, to eliminate foods with negative assay results and allow foods with positive assay results (comparison). The primary outcome was between-group differences in the IBS Global Improvement Scale (GIS). Secondary outcomes included reductions in IBS Symptom Severity Scale (SSS) scores and increases in IBS Adequate Relief (AR) and Quality of Life (QOL) scores. An aptamer-based proteomic analysis was conducted in strong responders.
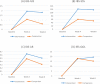
Results: The intervention group had significantly greater increases in mean GIS score after 4 weeks (0.86 vs comparison; 95% CI 0.05 to 1.67; p=0.04) and 8 weeks (1.22 vs comparison; 95% CI 0.22 to 2.22; p=0.02). The intervention group also had significantly greater reductions in mean SSS score at 4 weeks (-61.78 vs comparison; 95% CI -4.43 to -119.14; p=0.04) and 8 weeks (-66.42 vs comparison; 95% CI -5.75 to -127.09; p=0.03). There were no significant differences between intervention and comparison groups in mean AR or QOL scores. A reduction in neutrophil elastase concentration was associated with reduced symptoms.
Conclusions: Elimination diets guided by leucocyte activation tests reduced symptoms. These findings could lead to insights into the pathophysiology of IBS.
Trial registration number: NCT02186743.
Keywords: Dietary Factors; Irritable Bowel Syndrome; Quality Of Life.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Hungin AP, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther 2005;21:1365–75. doi:10.1111/j.1365-2036.2005.02463.x - DOI - PubMed
-
- Gaman A, Bucur MC, Kuo B. Therapeutic advances in functional gastrointestinal disease: irritable bowel syndrome. Therap Adv Gastroenterol 2009;2:169–81. doi:10.1177/1756283X08103656 - DOI - PMC - PubMed
-
- Simrén M, Månsson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001;63:108–15. doi:10.1159/000051878 - DOI - PubMed
-
- Gibson PR, Varney J, Malakar S, et al. Food components and irritable bowel syndrome. Gastroenterology 2015;148:1158–74. doi:10.1053/j.gastro.2015.02.005 - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
