Impact of swept source optical coherence tomography on ophthalmology
- PMID: 29018713
- PMCID: PMC5602691
- DOI: 10.1016/j.tjo.2015.09.002
Impact of swept source optical coherence tomography on ophthalmology
Abstract
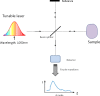
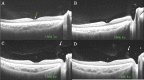
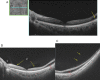
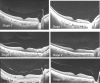
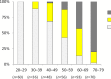
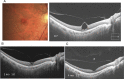
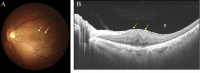
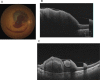
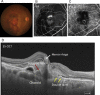
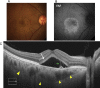
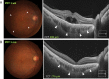
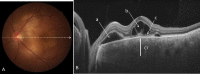
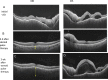
Swept source optical coherence tomography (SS-OCT) was introduced in clinical practice in 2012. Because of its deeper penetration and faster acquisition time, SS-OCT has the ability to visualize choroid, vitreous, and retinal structures behind dense preretinal hemorrhages. Swept source optical coherence tomography has positively influenced and hugely contributed to the research of the vitreous body. It is the first ophthalmic diagnostic technology to demonstrate the entire structure of the posterior precortical vitreous pocket (PPVP) in vivo. The roles of the PPVP in physiological posterior vitreous detachment and vitreoretinal interface disorders have now been elucidated. The presence of a connecting channel between the PPVP and Cloquet's canal suggests that the aqueous humor drains into the premacular space. Deeper penetration of SS-OCT has made it possible to view the choroid. It also has an important role in central serous chorioretinopathy and uveitis. We have also been able to treat Harada disease by monitoring the choroidal thickness by SS-OCT.
Keywords: Harada disease; macular hole; posterior precortical vitreous pocket; swept source optical coherence tomography; vitreoretinal interface disease.
Conflict of interest statement
Conflicts of interest: The author has no conflicts of interest to declare.
Figures





References
-
- Kishi S, Shimizu K. Posterior precortical vitreous pocket. Arch Ophthalmol. 1990;108:979–982. - PubMed
-
- Worst JG. Cisternal systems of the fully developed vitreous body in the young adult. Trans Ophthalmol Soc UK. 1977;97:550–554. - PubMed
-
- Worst J. Extracapsular surgery in lens implantation (Binkhorst lecture). Part IV. Some anatomical and pathophysiological implications. J Am Intraocul Implant Soc. 1978;4:7–14. - PubMed
-
- Kishi S, Shimizu K. Oval defect in detached posterior hyaloid membrane in idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1994;118:451–456. - PubMed
-
- Kishi S, Shimizu K. Clinical manifestations ofposterior precortical vitreous pocket in proliferative diabetic retinopathy. Ophthalmology. 1993;100:225–229. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

