Asthma Endotypes and an Overview of Targeted Therapy for Asthma
- PMID: 29018800
- PMCID: PMC5622943
- DOI: 10.3389/fmed.2017.00158
Asthma Endotypes and an Overview of Targeted Therapy for Asthma
Abstract
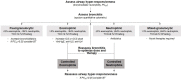
Guidelines for the management of severe asthma do not emphasize the measurement of the inflammatory component of airway disease to indicate appropriate treatments or to monitor response to treatment. Inflammation is a central component of asthma and contributes to symptoms, physiological, and structural abnormalities. It can be assessed by a number of endotyping strategies based on "omics" technology such as proteomics, transcriptomics, and metabolomics. It can also be assessed using simple cellular responses by quantitative cytometry in sputum. Bronchitis may be eosinophilic, neutrophilic, mixed-granulocytic, or paucigranulocytic (eosinophils and neutrophils not elevated). Eosinophilic bronchitis is usually a Type 2 (T2)-driven process and therefore a sputum eosinophilia of greater than 3% usually indicates a response to treatment with corticosteroids or novel therapies directed against T2 cytokines such as IL-4, IL-5, and IL-13. Neutrophilic bronchitis represents a non-T2-driven disease, which is generally a predictor of response to antibiotics and may be a predictor to therapies targeted at pathways that lead to neutrophil recruitment such as TNF, IL-1, IL-6, IL-8, IL-23, and IL-17. Paucigranulocytic disease may not warrant anti-inflammatory therapy. These patients, whose symptoms may be driven largely by airway hyper-responsiveness may benefit from smooth muscle-directed therapies such as bronchial thermoplasty or mast-cell directed therapies. This review will briefly summarize the current knowledge regarding "omics-based signatures" and cellular endotyping of severe asthma and give an overview of segmentation of asthma therapeutics guided by the endotype.
Keywords: endotype; omics; severe asthma; sputum cytometry; type 2-high asthma; type 2-low asthma.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources