Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies
- PMID: 29018908
- PMCID: PMC11028164
- DOI: 10.1007/s00262-017-2069-9
Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies
Abstract
Background: PD-1 antibodies (PD1ab) are increasingly used in metastatic melanoma and other malignancies. Arthralgia is an underestimated side effect of PD-1 antibody treatment with unknown cause. Our aim was to characterize PD1ab-induced arthralgia.
Patients and methods: We retrospectively included patients with metastatic cutaneous malignancies treated with pembrolizumab or nivolumab ± ipilimumab at the National Center for Tumor Diseases (Heidelberg) between 01/2013 and 09/2016. Arthralgia was characterized by laboratory diagnostics, imaging, and if indicated, rheumatologic consultation.
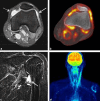
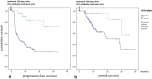
Results: 26 of 195 patients (13.3%) developed arthralgia. The median onset of symptoms was 100 days (7-780 days). Most frequently, arthralgia involved large joints (shoulders, knees) in a predominantly symmetrical pattern. Only two patients were seropositive for rheumatoid factor and/or anti-citrullinated protein antibodies. Ten patients developed the clinical picture of arthritis, with seven of them showing synovitis in MRI or PET/CT. Five patients showed inflammation in joints pre-damaged by osteoarthritis. In 11 patients arthralgia could not be specified. The majority of patients was satisfactorily treated with non-steroidal anti-inflammatory drugs (NSAIDs), 23.1% required additional low-dose corticosteroids and only 7.6% of our patients received further immunosuppressive treatment. Patients with arthralgia showed a better treatment response and improved PFS and OS.
Conclusion: Arthralgia is frequent during PD1ab treatment. The clinical picture varies between synovitis of predominantly large joints, progressive osteoarthritis and arthralgia without evident joint damage. Vast majority of cases can be satisfactorily managed by NSAID and/or low-dose corticosteroids.
Keywords: Arthralgia; Arthritis; Melanoma; Nivolumab; PD-1 antibody; Pembrolizumab.
Conflict of interest statement
K Buder-Bakhaya received honoraria and travel reimbursements from TEVA Pharmaceutical Industries GmbH, MSD Sharp & Dome GmbH Oncology (MSD), and Roche Pharma AG (Roche). K. Benesova received payment for lectures from Roche and Abbvie Germany GmbH & Co, KG (Abbvie), travel expenses and/or conference fees from Abbvie, Pfizer Pharma GmbH (Pfizer) and Bristol-Myers Squibb (BMS). H.-M. Lorenz received consultancy fees, honoraria for lectures, support for scientific projects or travel reimbursements from Abbvie, MSD, BMS, Pfizer, Roche, Celgene GmbH, Baxter Germany GmbH, Swedish Orphan Biovitrum GmbH, Biogen GmbH, Medac GmbH, GlaxoSmithKline GmbH & Co. KG (GSK), Chugai Pharma Europe Ltd., Novartis Pharma GmbH (Novartis), UCB Pharma GmbH, Janssen-Cilag GmbH, AstraZeneca GmbH, Lilly Germany GmbH, Actelion Pharmaceuticals Germany GmbH, Bayer Vital GmbH, Shire Germany GmbH, and Octapharm GmbH. A. Enk received consultancy fees and honoraria for lectures from Biotest AG, Galderma Laboratorium GmbH, Janssen-Cilag GmbH, AbbVie, BMS, MSD, and Roche. J.C. Hassel received consultancy fees from Amgen GmbH, and MSD, payment for lectures from BMS, MSD, Roche, GSK, Novartis, and Pfizer and travel reimbursements from BMS, MSD, Amgen, and GSK. All other authors declare that they have no conflicts of interest.
Figures
References
-
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, Investigators K Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. - DOI - PubMed
-
- Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. - DOI - PubMed
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. - DOI - PMC - PubMed
-
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. - DOI - PMC - PubMed
-
- National Institutes of Health (2010) Common terminology criteria for adverse events (CTCAE), Version 4.03. U.S. Department of Health and Human Services, Bethasda
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

