Physical Exercise Modulates L-DOPA-Regulated Molecular Pathways in the MPTP Mouse Model of Parkinson's Disease
- PMID: 29019056
- PMCID: PMC5994219
- DOI: 10.1007/s12035-017-0775-0
Physical Exercise Modulates L-DOPA-Regulated Molecular Pathways in the MPTP Mouse Model of Parkinson's Disease
Abstract
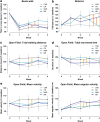
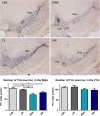
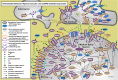
Parkinson's disease (PD) is characterized by the degeneration of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc), resulting in motor and non-motor dysfunction. Physical exercise improves these symptoms in PD patients. To explore the molecular mechanisms underlying the beneficial effects of physical exercise, we exposed 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine (MPTP)-treated mice to a four-week physical exercise regimen, and subsequently explored their motor performance and the transcriptome of multiple PD-linked brain areas. MPTP reduced the number of DA neurons in the SNpc, whereas physical exercise improved beam walking, rotarod performance, and motor behavior in the open field. Further, enrichment analyses of the RNA-sequencing data revealed that in the MPTP-treated mice physical exercise predominantly modulated signaling cascades that are regulated by the top upstream regulators L-DOPA, RICTOR, CREB1, or bicuculline/dalfampridine, associated with movement disorders, mitochondrial dysfunction, and epilepsy-related processes. To elucidate the molecular pathways underlying these cascades, we integrated the proteins encoded by the exercise-induced differentially expressed mRNAs for each of the upstream regulators into a molecular landscape, for multiple key brain areas. Most notable was the opposite effect of physical exercise compared to previously reported effects of L-DOPA on the expression of mRNAs in the SN and the ventromedial striatum that are involved in-among other processes-circadian rhythm and signaling involving DA, neuropeptides, and endocannabinoids. Altogether, our findings suggest that physical exercise can improve motor function in PD and may, at the same time, counteract L-DOPA-mediated molecular mechanisms. Further, we hypothesize that physical exercise has the potential to improve non-motor symptoms of PD, some of which may be the result of (chronic) L-DOPA use.
Keywords: (Non-)motor function; L-DOPA; MPTP; Molecular landscape; Parkinson’s disease; Physical exercise.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Figures



References
-
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. - PubMed
-
- Sveinbjornsdottir S. The clinical symptoms of Parkinson's disease. J Neurochem. 2016;139(Suppl 1):318–324. - PubMed
-
- Cotzias GC, Van Woert MH, Schiffer LM. Aromatic amino acids and modification of parkinsonism. N Engl J Med. 1967;276(7):374–379. - PubMed
-
- Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M, et al. The Movement Disorder Society evidence-based medicine review update: treatments for the motor symptoms of Parkinson's disease. Mov Disord. 2011;26(Suppl 3):S2–41. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

