Self-assembling prodrugs
- PMID: 29019492
- PMCID: PMC5844511
- DOI: 10.1039/c7cs00521k
Self-assembling prodrugs
Abstract
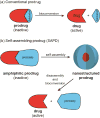
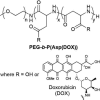
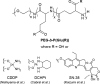
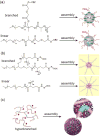
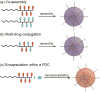
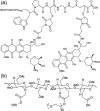
Covalent modification of therapeutic compounds is a clinically proven strategy to devise prodrugs with enhanced treatment efficacies. This prodrug strategy relies on the modified drugs that possess advantageous pharmacokinetic properties and administration routes over their parent drug. Self-assembling prodrugs represent an emerging class of therapeutic agents capable of spontaneously associating into well-defined supramolecular nanostructures in aqueous solutions. The self-assembly of prodrugs expands the functional space of conventional prodrug design, affording a possible pathway to more effective therapies as the assembled nanostructure possesses distinct physicochemical properties and interaction potentials that can be tailored to specific administration routes and disease treatment. In this review, we will discuss the various types of self-assembling prodrugs in development, providing an overview of the methods used to control their structure and function and, ultimately, our perspective on their current and future potential.
Figures
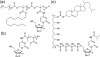
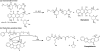


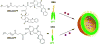
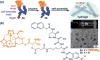

References
-
- Albert A. Nature. 1958;182:421–423. - PubMed
-
- Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Nature Reviews Drug Discovery. 2008;7:255–270. - PubMed
-
- Kevin B, Robert W, Iain G, Kevin D. Current Drug Metabolism. 2003;4:461–485. - PubMed
-
- Stella VJ, Nti-Addae KW. Advanced Drug Delivery Reviews. 2007;59:677–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

