Effects of Extremely Low Frequency Electromagnetic Fields on Melanogenesis through p-ERK and p-SAPK/JNK Pathways in Human Melanocytes
- PMID: 29019940
- PMCID: PMC5666802
- DOI: 10.3390/ijms18102120
Effects of Extremely Low Frequency Electromagnetic Fields on Melanogenesis through p-ERK and p-SAPK/JNK Pathways in Human Melanocytes
Abstract
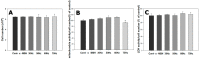
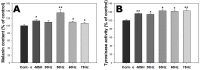
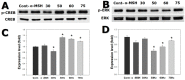
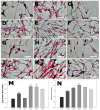
This study evaluated frequency-dependent effects of extremely low frequency electromagnetic fields (ELF-EMFs) on melanogenesis by melanocytes in vitro. Melanocytes were exposed to 2 mT EMFs at 30-75 Hz for 3 days before melanogenesis was examined. Exposure to ELF-EMFs at 50 and 60 Hz induced melanogenic maturation without cell damage, without changing cell proliferation and mitochondrial activity. Melanin content and tyrosinase activity of cells exposed to 50 Hz were higher than in controls, and mRNA expression of tyrosinase-related protein-2 was elevated relative to controls at 50 Hz. Phosphorylated cyclic adenosine monophosphate response element-binding protein (p-CREB) levels were higher than controls in cells exposed to ELF-EMFs at 50-75 Hz. Immunohistochemical staining showed that melanocyte-specific markers (HMB45, Melan-A) were strongly expressed in cells exposed to EMFs at 50 and 60 Hz compared to controls. Thus, exposure to ELF-EMFs at 50 Hz could stimulate melanogenesis in melanocytes, through activation of p-CREB and p-p38 and inhibition of phosphorylated extracellular signal-regulated protein kinase and phosphorylated stress-activated protein kinase/c-Jun N-terminal kinase. The results may form the basis of an appropriate anti-gray hair treatment or be applied in a therapeutic device for inducing repigmentation in the skin of vitiligo patients.
Keywords: MITF; extremely low frequency electromagnetic fields (ELF-EMFs); melanogenesis; p-CREB; tyrosinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



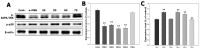


References
-
- Slominski A., Wortsman J., Luger T., Paus R., Solomon S. Corticotropin Releasing Hormone and Proopiomelanocortin Involvement in the Cutaneous Response to Stress. Physiol. Rev. 2000;80:979–1010. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

