Risk of Acute Liver Injury After Statin Initiation by Human Immunodeficiency Virus and Chronic Hepatitis C Virus Infection Status
- PMID: 29020184
- PMCID: PMC5850026
- DOI: 10.1093/cid/cix564
Risk of Acute Liver Injury After Statin Initiation by Human Immunodeficiency Virus and Chronic Hepatitis C Virus Infection Status
Abstract
Background: Patients with human immunodeficiency virus (HIV) and/or chronic hepatitis C virus (HCV) infection may be prescribed statins as treatment for metabolic/cardiovascular disease, but it remains unclear if the risk of acute liver injury (ALI) is increased for statin initiators compared to nonusers in groups classified by HIV/HCV status.
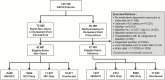
Methods: We conducted a cohort study to compare rates of ALI in statin initiators vs nonusers among 7686 HIV/HCV-coinfected, 8155 HCV-monoinfected, 17739 HIV-monoinfected, and 36604 uninfected persons in the Veterans Aging Cohort Study (2000-2012). We determined development of (1) liver aminotransferases >200 U/L, (2) severe ALI (coagulopathy with hyperbilirubinemia), and (3) death, all within 18 months. Cox regression was used to determine propensity score-adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) of outcomes in statin initiators compared to nonusers across the groups.
Results: Among HIV/HCV-coinfected patients, statin initiators had lower risks of aminotransferase levels >200 U/L (HR, 0.66 [95% CI, .53-.83]), severe ALI (HR, 0.23 [95% CI, .12-.46]), and death (HR, 0.36 [95% CI, .28-.46]) compared with statin nonusers. In the setting of chronic HCV alone, statin initiators had reduced risks of aminotransferase elevations (HR, 0.57 [95% CI, .45-.72]), severe ALI (HR, 0.15 [95% CI, .06-.37]), and death (HR, 0.42 [95% CI, .32-.54]) than nonusers. Among HIV-monoinfected patients, statin initiators had lower risks of aminotransferase increases (HR, 0.52 [95% CI, .40-.66]), severe ALI (HR, 0.26 [95% CI, .13-.55]), and death (HR, 0.19 [95% CI, .16-.23]) compared with nonusers. Results were similar among uninfected persons.
Conclusions: Regardless of HIV and/or chronic HCV status, statin initiators had a lower risk of ALI and death within 18 months compared with statin nonusers.
Keywords: HIV; acute liver injury; hepatitis C; hepatotoxicity; statins.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology 2004; 40:115–9. - PubMed
-
- Mehta SH, Brancati FL, Strathdee SA et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology 2003; 38:50–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

