Viral Surveillance in Serum Samples From Patients With Acute Liver Failure By Metagenomic Next-Generation Sequencing
- PMID: 29020199
- PMCID: PMC5848299
- DOI: 10.1093/cid/cix596
Viral Surveillance in Serum Samples From Patients With Acute Liver Failure By Metagenomic Next-Generation Sequencing
Abstract
Background: Twelve percent of all acute liver failure (ALF) cases are of unknown origin, often termed indeterminate. A previously unrecognized hepatotropic virus has been suspected as a potential etiologic agent.
Methods: We compared the performance of metagenomic next-generation sequencing (mNGS) with confirmatory nucleic acid testing (NAT) to routine clinical diagnostic testing in detection of known or novel viruses associated with ALF. Serum samples from 204 adult ALF patients collected from 1998 to 2010 as part of a nationwide registry were analyzed. One hundred eighty-seven patients (92%) were classified as indeterminate, while the remaining 17 patients (8%) served as controls, with infections by either hepatitis A virus or hepatitis B virus (HBV), or a noninfectious cause for their ALF.
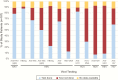
Results: Eight cases of infection from previously unrecognized viral pathogens were detected by mNGS (4 cases of herpes simplex virus type 1, including 1 case of coinfection with HBV, and 1 case each of HBV, parvovirus B19, cytomegalovirus, and human herpesvirus 7). Several missed dual or triple infections were also identified, and assembled viral genomes provided additional information on genotyping and drug resistance mutations. Importantly, no sequences corresponding to novel viruses were detected.
Conclusions: These results suggest that ALF patients should be screened for the presence of uncommon viruses and coinfections, and that most cases of indeterminate ALF in the United States do not appear to be caused by novel viral pathogens. In the future, mNGS testing may be useful for comprehensive diagnosis of viruses associated with ALF, or to exclude infectious etiologies.
Keywords: SURPI computational pipeline; indeterminate ALF; metagenomic next-generation sequencing; pathogen discovery; viral hepatitis.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
Viruses Associated With Unexplained Acute Liver Failure: Next Generation Reveals the Last Generation.Clin Infect Dis. 2017 Oct 16;65(9):1486-1488. doi: 10.1093/cid/cix597. Clin Infect Dis. 2017. PMID: 29020151 No abstract available.
References
-
- Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013; 369:2525–34. - PubMed
-
- Fagan EA. Acute liver failure of unknown pathogenesis: the hidden agenda. Hepatology 1994; 19:1307–12. - PubMed
-
- Lee WM, Brown KE, Young NS et al. ; Acute Liver Failure Study Group Brief report: no evidence for parvovirus B19 or hepatitis E virus as a cause of acute liver failure. Dig Dis Sci 2006; 51:1712–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

