Innate, T-, and B-Cell Responses in Acute Human Zika Patients
- PMID: 29020226
- PMCID: PMC5850027
- DOI: 10.1093/cid/cix732
Innate, T-, and B-Cell Responses in Acute Human Zika Patients
Abstract
Background: There is an urgent need for studies of viral persistence and immunity during human Zika infections to inform planning and conduct of vaccine clinical trials.
Methods: In 5 returned US travelers with acute symptomatic Zika infection, clinical features, viral RNA levels, and immune responses were characterized.
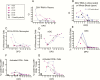
Results: Two pregnant, flavivirus-experienced patients had viral RNA persist in plasma for >44 and >26 days. Three days after symptom onset, transient increases in proinflammatory monocytes began followed at 5 days by transient decreases in myeloid dendritic cells. Anti-Zika virus immunoglobulin M was detected at day 7 after symptom onset, persisted beyond 103 days, and remained equivocal through day 172. Zika virus-specific plasmablasts and neutralizing antibodies developed quickly; dengue virus-specific plasmablasts and neutralizing antibodies at high titers developed only in flavivirus-experienced patients. Zika virus- and dengue virus-specific memory B cells developed in both flavivirus-naive and -experienced patients. CD4+ T cells were moderately activated and produced antiviral cytokines after stimulation with Zika virus C, prM, E, and NS5 peptides in 4/4 patients. In contrast, CD8+ T cells were massively activated, but virus-specific cells that produced cytokines were present in only 2/4 patients assessed.
Conclusions: Acute infections with Zika virus modulated antigen-presenting cell populations early. Flavivirus-experienced patients quickly recalled cross-reactive MBCs to secrete antibodies. Dengue virus-naive patients made little dengue-specific antibody but developed MBCs that cross-reacted against dengue virus. Zika virus-specific functional CD4+ T cells were readily detected, but few CD8+ T cells specific for the tested peptides were found.
Keywords: Zika; flavivirus; immunity; pregnancy; viral persistence.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



References
-
- Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases Zika Virus Reporting and Surveillance. Available at: https://www.cdc.gov/zika/reporting/case-counts.html. Accessed 16 May 2017.
-
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med 2016; 374:1981–7. - PubMed
-
- Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy—Brazil, 2015. Morb Mortal Wkly Rep 2016; 65:242–7. - PubMed
-
- Oehler E, Watrin L, Larre P et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill 2014; 19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

