Levofloxacin Prophylaxis During Induction Therapy for Pediatric Acute Lymphoblastic Leukemia
- PMID: 29020310
- PMCID: PMC5850441
- DOI: 10.1093/cid/cix644
Levofloxacin Prophylaxis During Induction Therapy for Pediatric Acute Lymphoblastic Leukemia
Abstract
Background: Infection is the most important cause of treatment-related morbidity and mortality in pediatric patients treated for acute lymphoblastic leukemia (ALL). Although routine in adults with leukemia, antibacterial prophylaxis is controversial in pediatrics because of insufficient evidence for its efficacy or antibiotic choice and concerns about promoting antibiotic resistance and Clostridium difficile infection.
Methods: This was a single-center, observational cohort study of patients with newly diagnosed ALL, comparing prospectively collected infection-related outcomes in patients who received no prophylaxis, levofloxacin prophylaxis, or other prophylaxis during induction therapy on the total XVI study. A propensity score-weighted logistic regression model was used to adjust for confounders.
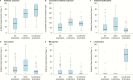
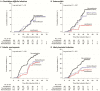
Results: Of 344 included patients, 173 received no prophylaxis, 69 received levofloxacin prophylaxis, and 102 received other prophylaxis regimens. Patients receiving prophylaxis had longer duration of neutropenia. Prophylaxis reduced the odds of febrile neutropenia, likely bacterial infection, and bloodstream infection by ≥70%. Levofloxacin prophylaxis alone reduced these infections, but it also reduced cephalosporin, aminoglycoside, and vancomycin exposure and reduced the odds of C. difficile infection by >95%. No increase in breakthrough infections with antibiotic-resistant organisms was seen, but this cannot be excluded.
Conclusions: This is the largest study to date of antibacterial prophylaxis during induction therapy for pediatric ALL and the first to include a broad-spectrum fluoroquinolone. Prophylaxis prevented febrile neutropenia and systemic infection. Levofloxacin prophylaxis also minimized the use of treatment antibiotics and drastically reduced C. difficile infection. Although long-term antibiotic-resistance monitoring is needed, these data support using targeted prophylaxis with levofloxacin in children undergoing induction chemotherapy for ALL.
Clinical trials registration: NCT00549848.
Keywords: Clostridium difficile; child; leukemia; levofloxacin; prophylaxis.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Effect of Levofloxacin Prophylaxis on Bacteremia in Children With Acute Leukemia or Undergoing Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial.JAMA. 2018 Sep 11;320(10):995-1004. doi: 10.1001/jama.2018.12512. JAMA. 2018. PMID: 30208456 Free PMC article. Clinical Trial.
-
Antibiotic prophylaxis and the gastrointestinal resistome in paediatric patients with acute lymphoblastic leukaemia: a cohort study with metagenomic sequencing analysis.Lancet Microbe. 2021 Apr;2(4):e159-e167. doi: 10.1016/s2666-5247(20)30202-0. Epub 2021 Feb 15. Lancet Microbe. 2021. PMID: 34355208 Free PMC article.
-
Feasibility, efficacy, and adverse effects of outpatient antibacterial prophylaxis in children with acute myeloid leukemia.Cancer. 2014 Jul 1;120(13):1985-92. doi: 10.1002/cncr.28688. Epub 2014 Mar 26. Cancer. 2014. PMID: 24677028 Free PMC article.
-
Fluoroquinolone Prophylaxis in Children With Cancer: A Pro/Con Discussion.J Pediatric Infect Dis Soc. 2024 Sep 26;13(9):486-492. doi: 10.1093/jpids/piae077. J Pediatric Infect Dis Soc. 2024. PMID: 39073450 Review.
-
Efficacy of levofloxacin as an antibacterial prophylaxis for acute leukemia patients receiving intensive chemotherapy: a systematic review and meta-analysis.Hematology. 2019 Dec;24(1):362-368. doi: 10.1080/16078454.2019.1589706. Hematology. 2019. PMID: 30880638
Cited by
-
Evaluation of Plasma Microbial Cell-Free DNA Sequencing to Predict Bloodstream Infection in Pediatric Patients With Relapsed or Refractory Cancer.JAMA Oncol. 2020 Apr 1;6(4):552-556. doi: 10.1001/jamaoncol.2019.4120. JAMA Oncol. 2020. PMID: 31855231 Free PMC article. Clinical Trial.
-
Effectiveness of Quinolone Prophylaxis in Pediatric Acute Leukemia and Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-analysis.Open Forum Infect Dis. 2022 Nov 3;9(12):ofac594. doi: 10.1093/ofid/ofac594. eCollection 2022 Dec. Open Forum Infect Dis. 2022. PMID: 36504701 Free PMC article. Review.
-
Early T-Cell Precursor Leukemia Has a Higher Risk of Induction-Related Infection among T-Cell Acute Lymphoblastic Leukemia in Adult.Mediators Inflamm. 2020 Dec 23;2020:8867760. doi: 10.1155/2020/8867760. eCollection 2020. Mediators Inflamm. 2020. PMID: 33424437 Free PMC article.
-
Antibiotic prophylaxis in acute childhood leukemia: What is known so far?Hematol Transfus Cell Ther. 2023 Oct-Dec;45(4):473-482. doi: 10.1016/j.htct.2022.09.1279. Epub 2022 Nov 21. Hematol Transfus Cell Ther. 2023. PMID: 36522273 Free PMC article. Review.
-
Bloodstream infections exacerbate incidence and severity of symptomatic glucocorticoid-induced osteonecrosis.Pediatr Blood Cancer. 2019 Jun;66(6):e27669. doi: 10.1002/pbc.27669. Epub 2019 Feb 13. Pediatr Blood Cancer. 2019. PMID: 30758124 Free PMC article.
References
-
- Christensen MS, Heyman M, Mottonen M et al. . Treatment-related death in childhood acute lymphoblastic leukaemia in the Nordic countries: 1992–2001. Br J Haematol 2005; 131:50–8. - PubMed
-
- O’Connor D, Bate J, Wade R et al. . Infection-related mortality in children with acute lymphoblastic leukemia: an analysis of infectious deaths on UKALL2003. Blood 2014; 124:1056–61. - PubMed
-
- Slats AM, Egeler RM, van der Does-van den Berg A et al. . Causes of death–other than progressive leukemia–in childhood acute lymphoblastic (ALL) and myeloid leukemia (AML): the Dutch Childhood Oncology Group experience. Leukemia 2005; 19:537–44. - PubMed
-
- Fiser RT, West NK, Bush AJ, Sillos EM, Schmidt JE, Tamburro RF. Outcome of severe sepsis in pediatric oncology patients. Pediatr Crit Care Med 2005; 6:531–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

