Vitamin D3 Supplementation Increases Spine Bone Mineral Density in Adolescents and Young Adults With Human Immunodeficiency Virus Infection Being Treated With Tenofovir Disoproxil Fumarate: A Randomized, Placebo-Controlled Trial
- PMID: 29020329
- PMCID: PMC5848310
- DOI: 10.1093/cid/cix753
Vitamin D3 Supplementation Increases Spine Bone Mineral Density in Adolescents and Young Adults With Human Immunodeficiency Virus Infection Being Treated With Tenofovir Disoproxil Fumarate: A Randomized, Placebo-Controlled Trial
Abstract
Background: Tenofovir disoproxil fumarate (TDF) decreases bone mineral density (BMD). We hypothesized that vitamin D3 (VITD3) would increase BMD in youth receiving TDF.
Methods: This was a randomized, double-blind, placebo-controlled trial of directly observed VITD3 vs placebo every 4 weeks for 48 weeks in youth aged 16-24 years with HIV, RNA load <200 copies/mL, taking TDF-containing combination antiretroviral therapy (TDF-cART) for ≥180 days. Participants (N = 214) received a daily multivitamin containing VITD3 400 IU and calcium 162 mg, plus monthly randomized VITD3 50000 IU (n = 109) or placebo (n = 105). Outcome was change from baseline to week 48 in lumbar spine BMD (LSBMD). Data presented are median (Q1, Q3).
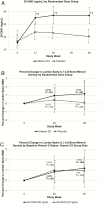
Results: Participants were aged 22.0 (21.0, 23.0) years, 84% were male, and 74% were black/African American. At baseline, 62% had 25-hydroxy vitamin D (25-OHD) <20 ng/mL. Multivitamin adherence was 49% (29%, 69%), and VITD3/placebo adherence 100% (100%, 100%). Vitamin D intake was 2020 (1914, 2168) and 284 (179, 394) IU/day, and serum 25-OHD concentration was 36.9 (30.5, 42.4) and 20.6 (14.4, 25.8) ng/mL at 48 weeks in VITD3 and placebo groups, respectively (P < .001). From baseline to week 48, LSBMD increased by 1.15% (-0.75% to 2.74%) in the VITD3 group (n = 99; P < .001) and 0.09% (-1.49% to 2.61%) in the placebo group (n = 89; P = .25), without between-group difference (P = .12). VITD3 group changes occurred with baseline 25-OHD <20 ng/mL (1.17% [-.82% to 2.90%]; P = .004) and ≥20 ng/mL (0.93% [-.26% to 2.15%]; P = .033).
Conclusions: For youth taking TDF-cART, LSBMD increased through 48 weeks with VITD3 plus multivitamin, but not with placebo plus multivitamin, independent of baseline vitamin D status.
Clinical trials registration: NCT01751646.
Keywords: HIV infection; bone mineral density; parathyroid hormone; tenofovir disoproxil fumarate; vitamin D supplementation.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr 2009; 51:554–61. - PubMed
-
- Gallant JE, Staszewski S, Pozniak AL et al. ; 903 Study Group Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292:191–201. - PubMed
-
- Moyle GJ, Stellbrink HJ, Compston J et al. ; ASSERT Team 96-week results of abacavir/lamivudine versus tenofovir/emtricitabine, plus efavirenz, in antiretroviral-naive, HIV-1-infected adults: ASSERT study. Antivir Ther 2013; 18:905–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

