Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae
- PMID: 29020404
- PMCID: PMC5850032
- DOI: 10.1093/cid/cix783
Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae
Abstract
Background: The efficacy of ceftazidime-avibactam-a cephalosporin-β-lactamase inhibitor combination with in vitro activity against Klebsiella pneumoniae carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CRE)-compared with colistin remains unknown.
Methods: Patients initially treated with either ceftazidime-avibactam or colistin for CRE infections were selected from the Consortium on Resistance Against Carbapenems in Klebsiella and other Enterobacteriaceae (CRACKLE), a prospective, multicenter, observational study. Efficacy, safety, and benefit-risk analyses were performed using intent-to-treat analyses with partial credit and the desirability of outcome ranking approaches. The ordinal efficacy outcome was based on disposition at day 30 after starting treatment (home vs not home but not observed to die in the hospital vs hospital death). All analyses were adjusted for confounding using inverse probability of treatment weighting (IPTW).
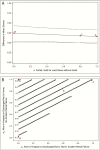
Results: Thirty-eight patients were treated first with ceftazidime-avibactam and 99 with colistin. Most patients received additional anti-CRE agents as part of their treatment. Bloodstream (n = 63; 46%) and respiratory (n = 30; 22%) infections were most common. In patients treated with ceftazidime-avibactam versus colistin, IPTW-adjusted all-cause hospital mortality 30 days after starting treatment was 9% versus 32%, respectively (difference, 23%; 95% bootstrap confidence interval, 9%-35%; P = .001). In an analysis of disposition at 30 days, patients treated with ceftazidime-avibactam, compared with those treated within colistin, had an IPTW-adjusted probability of a better outcome of 64% (95% confidence interval, 57%-71%). Partial credit analyses indicated uniform superiority of ceftazidime-avibactam to colistin.
Conclusions: Ceftazidime-avibactam may be a reasonable alternative to colistin in the treatment of K. pneumoniae carbapenemase-producing CRE infections. These findings require confirmation in a randomized controlled trial.
Keywords: Klebsiella pneumoniae; benefit-risk; carbapenem-resistant Enterobacteriaceae; ceftazidime-avibactam; colistin.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


References
-
- Tumbarello M, Trecarichi EM, De Rosa FG et al. ; ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 2015; 70:2133–43. - PubMed
-
- Falcone M, Russo A, Iacovelli A et al. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Microbiol Infect 2016; 22:444–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000439/TR/NCATS NIH HHS/United States
- R21 AI114508/AI/NIAID NIH HHS/United States
- UL1 TR002548/TR/NCATS NIH HHS/United States
- R01 AI063517/AI/NIAID NIH HHS/United States
- R01 AI119446/AI/NIAID NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 AI100560/AI/NIAID NIH HHS/United States
- UM1 AI104681/AI/NIAID NIH HHS/United States
- R21 AI107302/AI/NIAID NIH HHS/United States
- R01 AI104895/AI/NIAID NIH HHS/United States
- R01 AI072219/AI/NIAID NIH HHS/United States
- K24 AI093969/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

