Variability of intended copies for etanercept (Enbrel®): Data on multiple batches of seven products
- PMID: 29020508
- PMCID: PMC5800383
- DOI: 10.1080/19420862.2017.1387346
Variability of intended copies for etanercept (Enbrel®): Data on multiple batches of seven products
Abstract
Fusion protein and monoclonal antibody-based tumor necrosis factor (TNF) inhibitors represent established treatment options for a range of inflammatory diseases. Regulatory authorities have outlined the structural characterization and clinical assessments necessary to establish biosimilarity of a new biotherapeutic product with the innovator biologic drug. Biologic products that would not meet the minimum World Health Organization's standard for evaluation of similar biotherapeutic products are available in some countries; in some cases relevant data to assess biosimilarity and appropriate regulatory approval pathways are lacking. Batches of seven intended copy (IC) products for etanercept (Enbrel®) were subjected to a subset of test methods used in the routine release and heightened characterization of Enbrel®, to determine key attributes of identity, quality, purity, strength, and activity. While a number of quality attributes of the IC lots tested met the release specifications for Enbrel®, none fell within these limits across all methods performed, and there were no IC lots that satisfied the criteria typically applied by the innovator to support comparability with Enbrel®. Although the consequences of these differences are largely unknown, the potential for unanticipated clinical outcomes should not be overlooked.
Keywords: biosimilar; characterization; comparability; etanercept; intended copy; quality attribute.
Figures

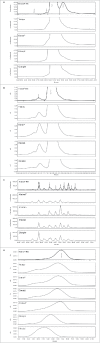
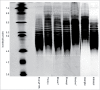
References
-
- Maksymowych WP, Dougados M, van der Heijde D, Sieper J, Braun J, Citera G, Van den Bosch F, Logeart I, Wajdula J, Jones H, et al.. Clinical and MRI responses to etanercept in early non-radiographic axial spondyloarthritis: 48-week results from the EMBARK study. Ann Rheum Dis. 2016;75:1328–35. doi: 10.1136/annrheumdis-2015-207596. PMID:26269397 - DOI - PMC - PubMed
-
- World Health Organization Guidelines on evaluation of similar biotherapeutic products (SBPs). 2010. [accessed 2016June23]. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEU....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
