Manufacturing history of etanercept (Enbrel®): Consistency of product quality through major process revisions
- PMID: 29020515
- PMCID: PMC5800370
- DOI: 10.1080/19420862.2017.1388483
Manufacturing history of etanercept (Enbrel®): Consistency of product quality through major process revisions
Abstract
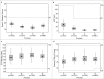
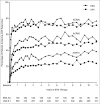
Etanercept (ETN) (Enbrel®) is a soluble protein that binds to, and specifically inhibits, tumor necrosis factor (TNF), a proinflammatory cytokine. ETN is synthesized in Chinese hamster ovary cells by recombinant DNA technology as a fusion protein, with a fully human TNFRII ectodomain linked to the Fc portion of human IgG1. Successful manufacture of biologics, such as ETN, requires sophisticated process and product understanding, as well as meticulous control of operations to maintain product consistency. The objective of this evaluation was to show that the product profile of ETN drug substance (DS) has been consistent over the course of production. Multiple orthogonal biochemical analyses, which included evaluation of attributes indicative of product purity, potency, and quality, were assessed on >2,000 batches of ETN from three sites of DS manufacture, during the period 1998-2015. Based on the key quality attributes of product purity (assessed by hydrophobic interaction chromatography HPLC), binding activity (to TNF by ELISA), potency (inhibition of TNF-induced apoptosis by cell-based bioassay) and quality (N-linked oligosaccharide map), we show that the integrity of ETN DS has remained consistent over time. This consistency was maintained through three major enhancements to the initial process of manufacturing that were supported by detailed comparability assessments, and approved by the European Medicines Agency. Examination of results for all major quality attributes for ETN DS indicates a highly consistent process for over 18 years and throughout changes to the manufacturing process, without affecting safety and efficacy, as demonstrated across a wide range of clinical trials of ETN in multiple inflammatory diseases.
Keywords: Etanercept; consistency; manufacturing; process; quality.
Figures

References
-
- European Medicines Agency Enbrel. Authorisation details. EMEA/H/C/000262. European Medicines Agency; 2000. February 2 [accessed 2017March22]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici....
-
- Pfizer Enbrel (etanercept) Summary of Product Characteristics. European Medicines Agency; 2010. February [accessed 2017March22]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info....
-
- Maksymowych WP, Dougados M, van der Heijde D, Sieper J, Braun J, Citera G, Van den Bosch F, Logeart I, Wajdula J, Jones H, et al.. Clinical and MRI responses to etanercept in early non-radiographic axial spondyloarthritis: 48-week results from the EMBARK study. Ann Rheum Dis. 2016;75:1328–35. doi:10.1136/annrheumdis-2015-207596. PMID:26269397. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
