Remarkable Stability of Myelinating Oligodendrocytes in Mice
- PMID: 29020619
- PMCID: PMC5643547
- DOI: 10.1016/j.celrep.2017.09.050
Remarkable Stability of Myelinating Oligodendrocytes in Mice
Abstract
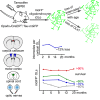
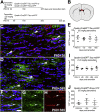
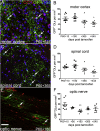
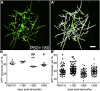
New myelin-forming oligodendrocytes (OLs) are generated in the mouse central nervous system during adulthood. These adult-born OLs might augment the existing population, contributing to neural plasticity, or else replace OLs that die in use (turnover). To distinguish between these alternatives, we induced genetic labeling of mature myelinating OLs in young adult mice and tracked their subsequent survival. OL survival rates were region dependent, being higher in corpus callosum (∼90% survival over 20 months) and motor cortex (∼70% survival) than in corticospinal tract or optic nerve (50%-60% survival). Survival rates over the first 8 months were 90%-100% in all regions except the optic nerve. In the corpus callosum, new OLs accumulate during young adulthood and are therefore likely to participate in adaptive myelination. We also found that the number of myelin internodes maintained by individual cortical OLs is stable for at least 8 months but declines ∼12% in the following year.
Keywords: Opalin-CreER; cell survival; cell turnover; cerebral cortex; corpus callosum; internode; myelin; optic nerve; spinal cord; transgenic mouse.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Dangata Y.Y., Kaufman M.H. Myelinogenesis in the optic nerve of (C57BL x CBA) F1 hybrid mice: a morphometric analysis. Eur. J. Morphol. 1997;35:3–17. - PubMed
-
- Danias J., Lee K.C., Zamora M.F., Chen B., Shen F., Filippopoulos T., Su Y., Goldblum D., Podos S.M., Mittag T. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57/BL6 mice. Invest. Ophthalmol. Vis. Sci. 2003;44:5151–5162. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

