Safety and Efficacy of AAV5 Vectors Expressing Human or Canine CNGB3 in CNGB3-Mutant Dogs
- PMID: 29020838
- PMCID: PMC5733651
- DOI: 10.1089/humc.2017.125
Safety and Efficacy of AAV5 Vectors Expressing Human or Canine CNGB3 in CNGB3-Mutant Dogs
Abstract
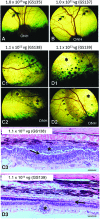
Achromatopsia is an inherited retinal disorder of cone photoreceptors characterized by markedly reduced visual acuity, extreme light sensitivity, and absence of color discrimination. Approximately 50% of cases are caused by mutations in the cone photoreceptor-specific cyclic nucleotide gated channel beta subunit (CNGB3) gene. Studies in CNGB3-mutant dogs showed that subretinal injection of an AAV vector expressing human CNGB3, which has 76% amino acid identity with canine CNGB3, driven by a 2.1 kb human red cone opsin promoter (PR2.1) and packaged in AAV5 capsids (AAV5-PR2.1-hCNGB3) rescued cone photoreceptor function, but at high doses was associated with an inflammatory response (focal chorioretinitis) consistent with immune-mediated toxicity. AAV vectors containing the PR2.1 promoter packaged in AAV5 capsids and expressing either the native canine CNGB3 (AAV5-PR2.1-cCNGB3) or the human CNGB3 (AAV5-PR2.1-hCNGB3) were evaluated at different dose levels in CNGB3-mutant dogs. The vector expressing canine CNGB3 achieved somewhat better rescue of cone function but unexpectedly was associated with a greater degree of retinal toxicity than the vector expressing human CNGB3. Very low-level T-cell immune responses to some AAV or CNGB3 peptides were observed in animals that received the higher vector dose. There was a more than twofold increase in serum neutralizing antibodies to AAV in one of three animals in the low-dose group and in two of three animals in the high-dose group. No serum anti-hCNGB3 antibodies were detected in any animal. The results of this study do not support the hypothesis that the focal chorioretinitis seen with high doses of AAV5-PR2.1-hCNGB3 in the initial studies was due to an immune response to human CNGB3.
Keywords: AAV; achromatopsia; chorioretinitis; gene therapy.
Conflict of interest statement
G.Y. and J.D.C. are employees and shareholders of AGTC and have a conflict of interest to the extent that this work potentially increases their financial interests. W.W.H. and the University of Florida have a financial interest in the use of AAV therapies. W.W.H. owns equity in and is a consultant for AGTC and has a conflict of interest to the extent that this work potentially increases his financial interests. No competing financial interests exist for the remaining authors.
Figures


References
-
- Kohl S, Jagle H, Sharpe LT, et al. . Achromatopsia. In: Pagon RA, Bird TC, Dolan CR, Stephens K, eds. Gene Reviews [Internet]. Seattle, WA: University of Washington
-
- Sharpe LT, Stockman A, Jagle H, et al. . Opsin genes, cone photopigments, color vision, and color blindness: rod monochromacy. In: Gegenfurtner K, Sharpe LT, eds. Color Vision: From Genes to Perception. Cambridge, United Kingdom: Cambridge University Press, 1999:48–51
-
- Kohl S, Varsanyi B, Antunes GA, et al. . CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet 2005;13:302–308 - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

