Blood-Based Protein Biomarkers for the Management of Traumatic Brain Injuries in Adults Presenting to Emergency Departments with Mild Brain Injury: A Living Systematic Review and Meta-Analysis
- PMID: 29020853
- PMCID: PMC8054517
- DOI: 10.1089/neu.2017.5182
Blood-Based Protein Biomarkers for the Management of Traumatic Brain Injuries in Adults Presenting to Emergency Departments with Mild Brain Injury: A Living Systematic Review and Meta-Analysis
Abstract
Accurate diagnosis of traumatic brain injury (TBI) is critical to effective management and intervention, but can be challenging in patients with mild TBI. A substantial number of studies have reported the use of circulating biomarkers as signatures for TBI, capable of improving diagnostic accuracy and clinical decision making beyond current practice standards. We performed a systematic review and meta-analysis to comprehensively and critically evaluate the existing body of evidence for the use of blood protein biomarkers (S100 calcium binding protein B [S100B], glial fibrillary acidic protein [GFAP], neuron specific enolase [NSE], ubiquitin C-terminal hydrolase-L1 [UCH-L1]. tau, and neurofilament proteins) for diagnosis of intracranial lesions on CT following mild TBI. Effects of potential confounding factors and differential diagnostic performance of the included markers were explored. Further, appropriateness of study design, analysis, quality, and demonstration of clinical utility were assessed. Studies published up to October 2016 were identified through searches of MEDLINE®, Embase, EBM Reviews, the Cochrane Library, World Health Organization (WHO), International Clinical Trials Registry Platform (ICTRP), and clinicaltrials.gov. Following screening of the identified articles, 26 were selected as relevant. We found that measurement of S100B can help informed decision making in the emergency department, possibly reducing resource use; however, there is insufficient evidence that any of the other markers is ready for clinical application. Our work pointed out serious problems in the design, analysis, and reporting of many of the studies, and identified substantial heterogeneity and research gaps. These findings emphasize the importance of methodologically rigorous studies focused on a biomarker's intended use, and defining standardized, validated, and reproducible approaches. The living nature of this systematic review, which will summarize key updated information as it becomes available, can inform and guide future implementation of biomarkers in the clinical arena.
Keywords: TBI; biomarkers; diagnosis; living systematic review; meta-analysis.
Conflict of interest statement
Dr. Wang is a former employee of Banyan Biomarkers Inc. and owns stock. Dr. Wang also receives royalties from licensing fees, and as such, Dr. Wang may benefit financially as a result of the outcomes of this research or work reported in this publication. There are no other disclosures to report.
Figures
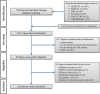





References
-
- Roozenbeek, B., Maas, A.I., and Menon, D.K. (2013). Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 9, 231–236 - PubMed
-
- Katz, D.I., Cohen, S.I., and Alexander, M.P. (2015). Mild traumatic brain injury. Handb. Clin. Neurol. 127, 131–156 - PubMed
-
- Biberthaler, P., Linsenmeier, U., Pfeifer, K.J., Kroetz, M., Mussack, T., Kanz, K.G., Hoecherl, E.F., Jonas, F., Marzi, I., Leucht, P., Jochum, M., and Mutschler, W. (2006). Serum S-100B concentration provides additional information fot the indication of computed tomography in patients after minor head injury: a prospective multicenter study. Shock 25, 446–453 - PubMed
-
- Haydel, M.J., Preston, C.A., Mills, T.J., Luber, S., Blaudeau, E., and DeBlieux, P.M. (2000). Indications for computed tomography in patients with minor head injury. N. Engl. J. Med. 343, 100–105 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
