The molecular mechanism of N-acetylglucosamine side-chain attachment to the Lancefield group A carbohydrate in Streptococcus pyogenes
- PMID: 29021255
- PMCID: PMC5702681
- DOI: 10.1074/jbc.M117.815910
The molecular mechanism of N-acetylglucosamine side-chain attachment to the Lancefield group A carbohydrate in Streptococcus pyogenes
Abstract
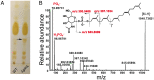
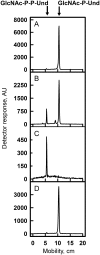
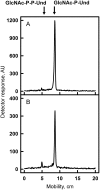
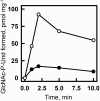
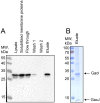
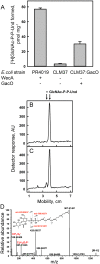
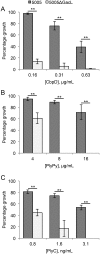
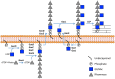
In many Lactobacillales species (i.e. lactic acid bacteria), peptidoglycan is decorated by polyrhamnose polysaccharides that are critical for cell envelope integrity and cell shape and also represent key antigenic determinants. Despite the biological importance of these polysaccharides, their biosynthetic pathways have received limited attention. The important human pathogen, Streptococcus pyogenes, synthesizes a key antigenic surface polymer, the Lancefield group A carbohydrate (GAC). GAC is covalently attached to peptidoglycan and consists of a polyrhamnose polymer, with N-acetylglucosamine (GlcNAc) side chains, which is an essential virulence determinant. The molecular details of the mechanism of polyrhamnose modification with GlcNAc are currently unknown. In this report, using molecular genetics, analytical chemistry, and mass spectrometry analysis, we demonstrated that GAC biosynthesis requires two distinct undecaprenol-linked GlcNAc-lipid intermediates: GlcNAc-pyrophosphoryl-undecaprenol (GlcNAc-P-P-Und) produced by the GlcNAc-phosphate transferase GacO and GlcNAc-phosphate-undecaprenol (GlcNAc-P-Und) produced by the glycosyltransferase GacI. Further investigations revealed that the GAC polyrhamnose backbone is assembled on GlcNAc-P-P-Und. Our results also suggested that a GT-C glycosyltransferase, GacL, transfers GlcNAc from GlcNAc-P-Und to polyrhamnose. Moreover, GacJ, a small membrane-associated protein, formed a complex with GacI and significantly stimulated its catalytic activity. Of note, we observed that GacI homologs perform a similar function in Streptococcus agalactiae and Enterococcus faecalis In conclusion, the elucidation of GAC biosynthesis in S. pyogenes reported here enhances our understanding of how other Gram-positive bacteria produce essential components of their cell wall.
Keywords: Streptococcus pyogenes (S. pyogenes); carbohydrate biosynthesis; cell wall; glycosyltransferase; lipid intermediate; polysaccharide.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Carapetis J. R., Steer A. C., Mulholland E. K., and Weber M. (2005) The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5, 685–694 - PubMed
-
- van Sorge N. M., Cole J. N., Kuipers K., Henningham A., Aziz R. K., Kasirer-Friede A., Lin L., Berends E. T. M., Davies M. R., Dougan G., Zhang F., Dahesh S., Shaw L., Gin J., Cunningham M., et al. (2014) The classical lancefield antigen of group a Streptococcus is a virulence determinant with implications for vaccine design. Cell Host Microbe 15, 729–740 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

