Prying into the Prion Hypothesis for Parkinson's Disease
- PMID: 29021298
- PMCID: PMC5637113
- DOI: 10.1523/JNEUROSCI.1788-16.2017
Prying into the Prion Hypothesis for Parkinson's Disease
Abstract
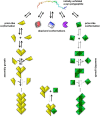
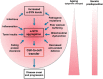
In Parkinson's disease, intracellular α-synuclein inclusions form in neurons. We suggest that prion-like behavior of α-synuclein is a key component in Parkinson's disease pathogenesis. Although multiple molecular changes are involved in the triggering of the disease process, we propose that neuron-to-neuron transfer is a crucial event that is essential for Lewy pathology to spread from one brain region to another. In this review, we describe key findings in human postmortem brains, cultured cells, and animal models of disease that support the idea that α-synuclein can act as a prion. We consider potential triggers of the α-synuclein misfolding and why the aggregates escape cellular degradation under disease conditions. We also discuss whether different strains of α-synuclein fibrils can underlie differences in cellular and regional distribution of aggregates in different synucleinopathies. Our conclusion is that α-synuclein probably acts as a prion in human diseases, and a deeper understanding of this step in the pathogenesis of Parkinson's disease can facilitate the development of disease-modifying therapies in the future.Dual Perspectives Companion Paper: Parkinson's Disease Is Not Simply a Prion Disorder, by D. James Surmeier, José A. Obeso, and Glenda M. Halliday.
Keywords: Lewy body; alpha-synuclein; neurodegenerative disease; propagation; seeding.
Copyright © 2017 the authors 0270-6474/17/379808-11$15.00/0.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
