Quiescence Promotes Latent HIV Infection and Resistance to Reactivation from Latency with Histone Deacetylase Inhibitors
- PMID: 29021396
- PMCID: PMC5709582
- DOI: 10.1128/JVI.01080-17
Quiescence Promotes Latent HIV Infection and Resistance to Reactivation from Latency with Histone Deacetylase Inhibitors
Abstract
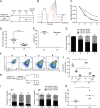
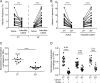
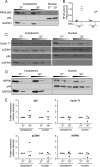
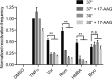
Human immunodeficiency virus type 1 (HIV-1) establishes transcriptionally silent latent infections in resting memory T cells and hematopoietic stem and progenitor cells (HSPCs), which allows the virus to persist in infected individuals despite antiretroviral therapy. Developing in vitro models of HIV-1 latency that recapitulate the characteristics of latently infected cells in vivo is crucial to identifying and developing effective latency-reversing therapies. HSPCs exist in a quiescent state in vivo, and quiescence is correlated with latent infections in T cells. However, current models for culturing HSPCs and for infecting T cells in vitro require that the cells be maintained in an actively proliferating state. Here we describe a novel culture system in which primary human HSPCs cultured under hypothermic conditions are maintained in a quiescent state. We show that these quiescent HSPCs are susceptible to predominantly latent infection with HIV-1, while actively proliferating and differentiating HSPCs obtain predominantly active infections. Furthermore, we demonstrate that the most primitive quiescent HSPCs are more resistant to spontaneous reactivation from latency than more differentiated HSPCs and that quiescent HSPCs are resistant to reactivation by histone deacetylase inhibitors or P-TEFb activation but are susceptible to reactivation by protein kinase C (PKC) agonists. We also demonstrate that inhibition of HSP90, a known regulator of HIV transcription, recapitulates the quiescence and latency phenotypes of hypothermia, suggesting that hypothermia and HSP90 inhibition may regulate these processes by similar mechanisms. In summary, these studies describe a novel model for studying HIV-1 latency in human primary cells maintained in a quiescent state.IMPORTANCE Human immunodeficiency virus type 1 (HIV-1) establishes a persistent infection for which there remains no feasible cure. Current approaches are unable to clear the virus despite decades of therapy due to the existence of latent reservoirs of integrated HIV-1, which can reactivate and contribute to viral rebound following treatment interruption. Previous clinical attempts to reactivate the latent reservoirs in an individual so that they can be eliminated by the immune response or viral cytopathic effect have failed, indicating the need for a better understanding of the processes regulating HIV-1 latency. Here we characterize a novel in vitro model of HIV-1 latency in primary hematopoietic stem and progenitor cells isolated from human cord blood that may better recapitulate the behavior of latently infected cells in vivo This model can be used to study mechanisms regulating latency and potential therapeutic approaches to reactivate latent infections in quiescent cells.
Keywords: 17-AAG; HIV; HSP90; NF-κB; P-TEFb; bryostatin; histone deacetylase inhibitors; latency; quiescence; stem cells.
Copyright © 2017 American Society for Microbiology.
Figures




References
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5:512–517. doi: 10.1038/8394. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

