Human TAUP301L overexpression results in TAU hyperphosphorylation without neurofibrillary tangles in adult zebrafish brain
- PMID: 29021554
- PMCID: PMC5636889
- DOI: 10.1038/s41598-017-13311-5
Human TAUP301L overexpression results in TAU hyperphosphorylation without neurofibrillary tangles in adult zebrafish brain
Abstract
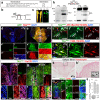
Microtubule-associated TAU protein is a pathological hallmark in Alzheimer's disease (AD), where hyperphosphorylation of TAU generates neurofibrillary tangles. To investigate the effects of TAU in a regenerative adult vertebrate brain system, we generated a cre/lox-based transgenic model of zebrafish that chronically expresses human TAUP301L, which is a variant of human TAU protein that forms neurofibrillary tangles in mouse models and humans. Interestingly, we found that although chronic and abundant expression of TAUP301L starting from early embryonic development led to hyperphosphorylation, TAUP301L did not form oligomers and neurofibrillary tangles, and did not cause elevated apoptosis and microglial activation, which are classical symptoms of tauopathies in mammals. Additionally, TAUP301L neither increased neural stem cell proliferation nor activated the expression of regenerative factor Interleukin-4, indicating that TAUP301L toxicity is prevented in the adult zebrafish brain. By combining TAUP301L expression with our established Aβ42 toxicity model, we found that Aβ42 ceases to initiate neurofibrillary tangle formation by TAUP301L, and TAUP301L does not exacerbate the toxicity of Aβ42. Therefore, our results propose a cellular mechanism that protects the adult zebrafish brain against tauopathies, and our model can be used to understand how TAU toxicity can be prevented in humans.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



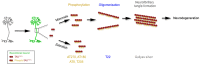
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

