Enhanced cell attachment and hemocompatibility of titanium by nanoscale surface modification through severe plastic integration of magnesium-rich islands and porosification
- PMID: 29021589
- PMCID: PMC5636805
- DOI: 10.1038/s41598-017-13169-7
Enhanced cell attachment and hemocompatibility of titanium by nanoscale surface modification through severe plastic integration of magnesium-rich islands and porosification
Abstract
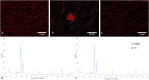
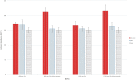
Besides the wide applications of titanium and its alloys for orthopedic and biomedical implants, the biocompatible nature of titanium has emerged various surface modification techniques to enhance its bioactivity and osteointegration with living tissues. In this work, we present a new procedure for nanoscale surface modification of titanium implants by integration of magnesium-rich islands combined with controlled formation of pores and refinement of the surface grain structure. Through severe plastic deformation of the titanium surface with fine magnesium hydride powder, Mg-rich islands with varying sizes ranging from 100 nm to 1000 nm can be integrated inside a thin surface layer (100-500 µm) of the implant. Selective etching of the surface forms a fine structure of surface pores which their average size varies in the range of 200-500 nm depending on the processing condition. In vitro biocompatibility and hemocompatibility assays show that the Mg-rich islands and the induced surface pores significantly enhance cell attachment and biocompatibility without an adverse effect on the cell viability. Therefore, severe plastic integration of Mg-rich islands on titanium surface accompanying with porosification is a new and promising procedure with high potential for nanoscale modification of biomedical implants.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

