Diffusion tensor imaging for multilevel assessment of the visual pathway: possibilities for personalized outcome prediction in autoimmune disorders of the central nervous system
- PMID: 29021839
- PMCID: PMC5607151
- DOI: 10.1007/s13167-017-0102-x
Diffusion tensor imaging for multilevel assessment of the visual pathway: possibilities for personalized outcome prediction in autoimmune disorders of the central nervous system
Abstract
The afferent visual pathway represents the most frequently affected white matter pathway in multiple sclerosis (MS) and neuromyelitis optica spectrum disorders (NMOSD). Diffusion tensor imaging (DTI) can reveal microstructural or non-overt brain tissue damage and quantify pathological processes. DTI facilitates the reconstruction of major white matter fiber tracts allowing for the assessment of structure-function and damage-dysfunction relationships. In this review, we outline DTI studies investigating the afferent visual pathway in idiopathic optic neuritis (ON), NMOSD, and MS. Since MS damage patterns are believed to depend on multiple factors, i.e., ON (anterior visual pathway damage), inflammatory lesions (posterior visual pathway damage), and global diffuse inflammatory and neurodegenerative processes, comprehensive knowledge on different contributing factors using DTI in vivo may advance our understanding of MS disease pathology. Combination of DTI measures and visual outcome parameters yields the potential to improve routine clinical diagnostic procedures and may further the accuracy of individual prognosis with regard to visual function and personalized disease outcome. However, due to the inherent limitations of DTI acquisition and post-processing techniques and the so far heterogeneous and equivocal data of previous studies, evaluation of the true potential of DTI as a possible biomarker for afferent visual pathway dysfunction is still substantially limited. Further research efforts with larger longitudinal studies and standardized DTI acquisition and post-processing validation criteria are needed to overcome current DTI limitations. DTI evaluation at different levels of the visual pathway has the potential to provide markers for individual damage evaluation in the future. As an imaging biomarker, DTI may support individual outcome prediction during personalized treatment algorithms in MS and other neuroinflammatory diseases, hereby leveraging the concept of predictive, preventive, and personalized medicine in the field of clinical neuroimmunology.
Keywords: DTI; Diffusion tensor imaging; Multiple sclerosis; Neuromyelitis optica spectrum disorders; Optic neuritis; Predictive preventive personalized medicine; Visual pathway.
Conflict of interest statement
Conflicts of interest
JK received conference registration fees from Biogen and financial research support from Krankheitsbezogenes Kompetenznetzwerk Multiple Sklerose (KKNMS), not related to this work. AUB served on the scientific advisory board for Biogen; received travel funding and/or speaker honoraria from Novartis and Biogen; has patents pending from Method and System for Optic Nerve Head Shape Quantification, perceptive visual computing based postural control analysis, multiple sclerosis biomarker, and perceptive sleep motion analysis; consulted for Nexus and Motognosis; and received research support from Novartis Pharma, Biogen Idec, BMWi, BMBF, and Guthy Jackson Charitable Foundation. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was funded by the authors. FP serves on the scientific advisory board for Novartis; received speaker honoraria and travel funding from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, and Shire; is an academic editor for PLoS One, is an associate editor for Neurology® Neuroimmunology & Neuroinflammation; consulted for Sanofi-Genzyme, Biogen Idec, MedImmune, Shire, and Alexion; received research support from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Alexion, Merck Serono, German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research, Arthur Arnstein Stiftung Berlin, EU FP7 Framework Program, Arthur Arnstein Foundation Berlin, Guthy Jackson Charitable Foundation, and National Multiple Sclerosis of the USA. MS states no conflicts of interest.
Funding
This study was funded by NeuroCure Cluster of Excellence (DFG Exc 257), Neu2 funding, and EXIST program.
Figures


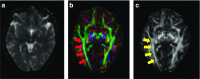
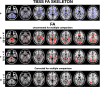
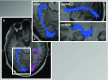
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

