Tumor-induced osteomalacia
- PMID: 29021995
- PMCID: PMC5633085
- DOI: 10.1016/j.bonr.2017.09.002
Tumor-induced osteomalacia
Abstract
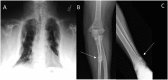
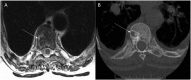
Tumor-induced osteomalacia (TIO) is a rare paraneoplastic syndrome clinically characterized by bone pain, fractures and muscle weakness. It is caused by tumoral overproduction of fibroblast growth factor 23 (FGF23) that acts primarily at the proximal renal tubule, decreasing phosphate reabsorption and 1α-hydroxylation of 25 hydroxyvitamin D, thus producing hypophosphatemia and osteomalacia. Lesions are typically small, benign mesenchymal tumors that may be found in bone or soft tissue, anywhere in the body. In up to 60% of these tumors, a fibronectin-1(FN1) and fibroblast growth factor receptor-1 (FGFR1) fusion gene has been identified that may serve as a tumoral driver. The diagnosis is established by the finding of acquired chronic hypophosphatemia due to isolated renal phosphate wasting with concomitant elevated or inappropriately normal blood levels of FGF23 and decreased or inappropriately normal 1,25-OH2-Vitamin D (1,25(OH)2D). Locating the tumor is critical, as complete removal is curative. For this purpose, a step-wise approach is recommended, starting with a thorough medical history and physical examination, followed by functional imaging. Suspicious lesions should be confirmed by anatomical imaging, and if needed, selective venous sampling with measurement of FGF23. If the tumor is not localized, or surgical resection is not possible, medical therapy with phosphate and active vitamin D is usually successful in healing the osteomalacia and reducing symptoms. However, compliance is often poor due to the frequent dosing regimen and side effects. Furthermore, careful monitoring is needed to avoid complications such us secondary/tertiary hyperparathyroidism, hypercalciuria, and nephrocalcinosis. Novel therapeutical approaches are being developed for TIO patients, such as image-guided tumor ablation and medical treatment with the anti-FGF23 monoclonal antibody KRN23 or anti FGFR medications. The case of a patient with TIO is presented to illustrate the importance of adequate and appropriate evaluation of patients with bone pain and hypophosphatemia, as well as an step-wise localization study of patients with suspected TIO.
Keywords: 1,25-OH2-vitamin D, 1,25(OH)2D; CT, computerized tomography; FDG-PET/CT, fluorodeoxyglucose positron emission tomography with computerized tomography; FGF1, fibroblast growth factor 1; FGF23; FGF23, fibroblast growth factor 23; FGFR1, fibroblast growth factor receptor-1; FISH, fluorescence in situ hybridization; FN1, fibronectin-1; MAPK, mitogen-activated protein kinase; MRI, magnetic resonance imaging; PMT, phosphaturic mesenchymal tumor; PTH, parathyroid hormone; Phosphaturic mesenchymal tumors; SPECT, single-photon emission computed tomography; TIO, tumor-induced osteomalacia; TRP, tubular reabsorption of phosphate; TmP/GFR, tubular maximum reabsorption of phosphate to glomerular filtration rate; Tumor-induced osteomalacia.
Figures





References
-
- Alon U., Hellerstein S. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr. Nephrol. 1994;8(2):250–251. - PubMed
-
- Aono Y., Hasegawa H., Yamazaki Y., Shimada T., Fujita T., Yamashita T., Fukumoto S. Anti-FGF-23 neutralizing antibodies ameliorate muscle weakness and decreased spontaneous movement of Hyp mice. J. Bone Miner. Res. 2011;26(4):803–810. - PubMed
-
- Berglund R.G.J., Forsberg J., Molinolo A., Fernandez de Castro L., Ten Hagen K., Tian E., Metwally T., Ovejero Crespo D., Chong W.H., Collins M.T. Insight into the molecular and cellular etiology of the tumors responsible for tumor-induced osteomalacia. Ann. Meet. Endocr. Soc. 2017:2017. (OR07–7)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

