Placental extract ameliorates non-alcoholic steatohepatitis (NASH) by exerting protective effects on endothelial cells
- PMID: 29022011
- PMCID: PMC5629350
- DOI: 10.1016/j.heliyon.2017.e00416
Placental extract ameliorates non-alcoholic steatohepatitis (NASH) by exerting protective effects on endothelial cells
Abstract
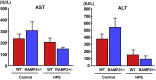
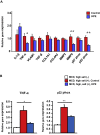
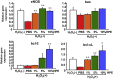
Non-alcoholic steatohepatitis (NASH) is a severe form of fatty liver disease that is defined by the presence of inflammation and fibrosis, ultimately leading to cirrhosis and hepatocellular carcinoma. Treatment with human placental extract (HPE) reportedly ameliorates the hepatic injury. We evaluated the effect of HPE treatment in a mouse model of NASH. In the methione- and choline-deficient (MCD) diet-induced liver injury model, fibrosis started from regions adjacent to the sinusoids. We administered the MCD diet with high-salt loading (8% NaCl in the drinking water) to mice deficient in the vasoprotective molecule RAMP2 for 5 weeks, with or without HPE. In both the HPE and control groups, fibrosis was seen in regions adjacent to the sinusoids, but the fibrosis was less pronounced in the HPE-treated mice. Levels of TNF-α and MMP9 expression were also significantly reduced in HPE-treated mice, and oxidative stress was suppressed in the perivascular region. In addition, HPE dose-dependently increased survival of cultured endothelial cells exposed to 100 μM H2O2, and it upregulated expression of eNOS and the anti-apoptotic factors bcl-2 and bcl-xL. From these observations, we conclude that HPE ameliorates NASH-associated pathologies by suppressing inflammation, oxidative stress and fibrosis. These beneficially effects of HPE are in part attributable to its protective effects on liver sinusoidal endothelial cells. HPE could thus be an attractive therapeutic candidate with which to suppress progression from simple fatty liver to NASH.
Keywords: Health Sciences; Metabolism; Pathology; Pharmaceutical science.
Figures







References
-
- Arai T., Sakurai T., Kamiyoshi A., Ichikawa-Shindo Y., Iinuma N., Iesato Y. Induction of LYVE-1/stabilin-2-positive liver sinusoidal endothelial-like cells from embryoid bodies by modulation of adrenomedullin-RAMP2 signaling. Peptides. 2011;32:1855–1865. - PubMed
-
- Caldwell-Kenkel J.C., Currin R.T., Tanaka Y., Thurman R.G., Lemasters J.J. Reperfusion injury to endothelial cells following cold ischemic storage of rat livers. Hepatology. 1989;10:292–299. - PubMed
-
- Chen C.P., Tsai P.S., Huang C.J. Antiinflammation effect of human placental multipotent mesenchymal stromal cells is mediated by prostaglandin E2 via a myeloid differentiation primary response gene 88-dependent pathway. Anesthesiology. 2012;117:568–579. - PubMed
-
- Day C.P., James O.F. Steatohepatitis: a tale of two hits. Gastroenterology. 1998;114:842–845. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

