Understanding and Interrupting the Fischer Azaindolization Reaction
- PMID: 29022706
- PMCID: PMC5726400
- DOI: 10.1021/jacs.7b07518
Understanding and Interrupting the Fischer Azaindolization Reaction
Abstract
Experimental and computational studies pertaining to the Fischer azaindolization reaction are reported. These studies explain why pyridylhydrazines are poorly reactive in Fischer indolization reactions, in addition to the origin of hydrazine substituent effects. Additionally, an interrupted variant of Fischer azaindolization methodology is disclosed, which provides a synthetic entryway into fused azaindoline scaffolds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



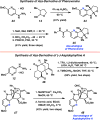
References
-
- Fischer E, Jourdan F. Ber. Dtsch. Chem. Ges. 1883;16:2241–2245.
- Fischer E, Hess O. Ber. Dtsch. Chem. Ges. 1884;17:559–568.
-
- Krüger S, Gaich T. Angew. Chem., Int. Ed. 2015;54:315–317. - PubMed
- Park J, Kim D-H, Das T, Cho C-G. Org. Lett. 2016;18:5098–5101. - PubMed
- Smith JM, Moreno J, Boal BW, Garg NK. J. Am. Chem. Soc. 2014;136:4504–4507. - PMC - PubMed
- Moreno J, Picazo E, Morrill LA, Smith JM, Garg NK. J. Am. Chem. Soc. 2016;138:1162–1165. - PMC - PubMed
- Ueda H, Satoh H, Matsumoto K, Sugimoto K, Fukuyama T, Tokuyama H. Angew. Chem., Int. Ed. 2009;48:7600–7603. - PubMed
- Satoh H, Ueda H, Tokuyama H. Tetrahedron. 2013;69:89–95.
- Wang D, Hou M, Ji Y, Gao S. Org. Lett. 2017;19:1922–1925. - PubMed
- Adams GL, Carroll PJ, Smith AB., III J. Am. Chem. Soc. 2012;134:4037–4040. - PMC - PubMed
- Adams GL, Carroll PJ, Smith AB., III J. Am. Chem. Soc. 2013;135:519–528. - PMC - PubMed
-
- Kelly AH, McLeod DH, Parrick J. Can. J. Chem. 1965;43:296–301.
- Kelly AH, Parrick J. Can. J. Chem. 1966;44:2455–2459.
- Crooks PA, Robinson B. Can. J. Chem. 1969;47:2061–2067.
- Jeanty M, Blu J, Suzenet F, Guillaumet G. Org. Lett. 2009;11:5142–5145. - PubMed
- Pin F, Buron F, Saab F, Colliandre L, Bourg S, Schoentgen F, Le Guevel R, Guillouzo C, Routier S. MedChemComm. 2011;2:899–903.
- Inman M, Carbone A, Moody CJ. J. Org. Chem. 2012;77:1217–1232. - PubMed
- Thomae D, Jeanty M, Coste J, Guillaumet G, Suzenet F. Eur. J. Org. Chem. 2013:3328–3336.
- Alekseyev RS, Amirova SR, Kabanova EV, Terenin VI. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2014;50:1305–1315.
-
- Perkin WH, Robinson R. J. Chem. Soc., Trans. 1913;103:1973–1985.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

