Zika virus infection dysregulates human neural stem cell growth and inhibits differentiation into neuroprogenitor cells
- PMID: 29022904
- PMCID: PMC5682681
- DOI: 10.1038/cddis.2017.517
Zika virus infection dysregulates human neural stem cell growth and inhibits differentiation into neuroprogenitor cells
Abstract
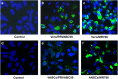
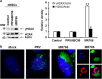
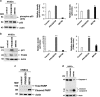
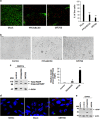
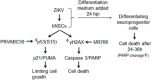
The current outbreak of Zika virus-associated diseases in South America and its threat to spread to other parts of the world has emerged as a global health emergency. A strong link between Zika virus and microcephaly exists, and the potential mechanisms associated with microcephaly are under intense investigation. In this study, we evaluated the effect of Zika virus infection of Asian and African lineages (PRVABC59 and MR766) in human neural stem cells (hNSCs). These two Zika virus strains displayed distinct infection pattern and growth rates in hNSCs. Zika virus MR766 strain increased serine 139 phosphorylation of histone H2AX (γH2AX), a known early cellular response proteins to DNA damage. On the other hand, PRVABC59 strain upregulated serine 15 phosphorylation of p53, p21 and PUMA expression. MR766-infected cells displayed poly (ADP-ribose) polymerase (PARP) and caspase-3 cleavage. Interestingly, infection of hNSCs by both strains of Zika virus for 24 h, followed by incubation in astrocyte differentiation medium, induced rounding and cell death. However, astrocytes generated from hNSCs by incubation in differentiation medium when infected with Zika virus displayed minimal cytopathic effect at an early time point. Infected hNSCs incubated in astrocyte differentiating medium displayed PARP cleavage within 24-36 h. Together, these results showed that two distinct strains of Zika virus potentiate hNSC growth inhibition by different mechanisms, but both viruses strongly induce death in early differentiating neuroprogenitor cells even at a very low multiplicity of infection. Our observations demonstrate further mechanistic insights for impaired neuronal homeostasis during active Zika virus infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Differential Responses of Human Fetal Brain Neural Stem Cells to Zika Virus Infection.Stem Cell Reports. 2017 Mar 14;8(3):715-727. doi: 10.1016/j.stemcr.2017.01.008. Epub 2017 Feb 16. Stem Cell Reports. 2017. PMID: 28216147 Free PMC article.
-
ZIKA virus elicits P53 activation and genotoxic stress in human neural progenitors similar to mutations involved in severe forms of genetic microcephaly.Cell Death Dis. 2016 Oct 27;7(10):e2440. doi: 10.1038/cddis.2016.266. Cell Death Dis. 2016. PMID: 27787521 Free PMC article.
-
Strain-Dependent Consequences of Zika Virus Infection and Differential Impact on Neural Development.Viruses. 2018 Oct 9;10(10):550. doi: 10.3390/v10100550. Viruses. 2018. PMID: 30304805 Free PMC article.
-
The impact of Zika virus in the brain.Biochem Biophys Res Commun. 2017 Oct 28;492(4):603-607. doi: 10.1016/j.bbrc.2017.01.074. Epub 2017 Jan 17. Biochem Biophys Res Commun. 2017. PMID: 28108286 Review.
-
Zika Virus and the Metabolism of Neuronal Cells.Mol Neurobiol. 2019 Apr;56(4):2551-2557. doi: 10.1007/s12035-018-1263-x. Epub 2018 Jul 24. Mol Neurobiol. 2019. PMID: 30043260 Free PMC article. Review.
Cited by
-
Apoptosis during ZIKA Virus Infection: Too Soon or Too Late?Int J Mol Sci. 2022 Jan 24;23(3):1287. doi: 10.3390/ijms23031287. Int J Mol Sci. 2022. PMID: 35163212 Free PMC article. Review.
-
Comparative genomics, infectivity and cytopathogenicity of Zika viruses produced by acutely and persistently infected human hematopoietic cell lines.PLoS One. 2018 Sep 7;13(9):e0203331. doi: 10.1371/journal.pone.0203331. eCollection 2018. PLoS One. 2018. PMID: 30192813 Free PMC article.
-
Zika virus dysregulates the expression of astrocytic genes involved in neurodevelopment.PLoS Negl Trop Dis. 2021 Apr 23;15(4):e0009362. doi: 10.1371/journal.pntd.0009362. eCollection 2021 Apr. PLoS Negl Trop Dis. 2021. PMID: 33891593 Free PMC article.
-
Human microglial models to study host-virus interactions.Exp Neurol. 2023 May;363:114375. doi: 10.1016/j.expneurol.2023.114375. Epub 2023 Mar 11. Exp Neurol. 2023. PMID: 36907350 Free PMC article. Review.
-
Zika virus: epidemiology, clinical aspects, diagnosis, and control of infection.Eur J Clin Microbiol Infect Dis. 2018 Nov;37(11):2035-2043. doi: 10.1007/s10096-018-3354-z. Epub 2018 Aug 30. Eur J Clin Microbiol Infect Dis. 2018. PMID: 30167886 Review.
References
-
- Vogel G. Evidence grows for Zika virus as pregnancy danger. Science 2016; 351: 1123–1124. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

