Long non-coding RNA Myd88 promotes growth and metastasis in hepatocellular carcinoma via regulating Myd88 expression through H3K27 modification
- PMID: 29022910
- PMCID: PMC5682683
- DOI: 10.1038/cddis.2017.519
Long non-coding RNA Myd88 promotes growth and metastasis in hepatocellular carcinoma via regulating Myd88 expression through H3K27 modification
Erratum in
-
Correction: Long non-coding RNA Myd88 promotes growth and metastasis in hepatocellular carcinoma via regulating Myd88 expression through H3K27 modification.Cell Death Dis. 2024 Dec 3;15(12):876. doi: 10.1038/s41419-024-07101-x. Cell Death Dis. 2024. PMID: 39627189 Free PMC article. No abstract available.
Abstract
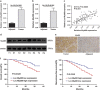
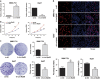
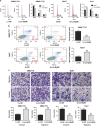
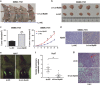
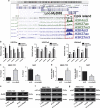
Enhanced Myd88 expression has been found in various parenchymal tumors especially in hepatocellular carcinoma with little mechanism of its upregulation known. A lot of long non-coding RNAs are reported to regulate the protein-coding genes which have location association through various mechanisms. In our study we confirmed a new long non-coding RNA Myd88 aberrant upregulated in HCC located upstream of Myd88 and verified a positive regulation relationship between them indicating that Lnc-Myd88 might participate in the enhanced expression of Myd88 in HCC. The gain- and loss-of-function analysis revealed that Lnc-Myd88 could promote the proliferation and metastasis of HCC both in vitro and in vivo. In addition, ChIP assays demonstrated that Lnc-Myd88 might increase Myd88 expression through enhancing H3K27Ac in the promoter of Myd88 gene, thus resulting in the activation of both NF-κB and PI3K/AKT signal pathways. In conclusion, we proposed that Lnc-Myd88 might serve as a novel diagnosis and therapeutic target for HCC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
LncRNA TRERNA1 facilitates hepatocellular carcinoma metastasis by dimethylating H3K9 in the CDH1 promoter region via the recruitment of the EHMT2/SNAI1 complex.Cell Prolif. 2019 Jul;52(4):e12621. doi: 10.1111/cpr.12621. Epub 2019 Apr 22. Cell Prolif. 2019. PMID: 31012192 Free PMC article.
-
Small nucleolar RNA ACA11 promotes proliferation, migration and invasion in hepatocellular carcinoma by targeting the PI3K/AKT signaling pathway.Biomed Pharmacother. 2017 Jun;90:705-712. doi: 10.1016/j.biopha.2017.04.014. Epub 2017 Apr 15. Biomed Pharmacother. 2017. PMID: 28419966
-
Myeloid differentiation factor 88 promotes growth and metastasis of human hepatocellular carcinoma.Clin Cancer Res. 2013 Jun 1;19(11):2905-16. doi: 10.1158/1078-0432.CCR-12-1245. Epub 2013 Apr 2. Clin Cancer Res. 2013. PMID: 23549880
-
The long non-coding RNA PTTG3P promotes cell growth and metastasis via up-regulating PTTG1 and activating PI3K/AKT signaling in hepatocellular carcinoma.Mol Cancer. 2018 May 26;17(1):93. doi: 10.1186/s12943-018-0841-x. Mol Cancer. 2018. PMID: 29803224 Free PMC article.
-
Upregulation of lncRNA FER1L4 suppresses the proliferation and migration of the hepatocellular carcinoma via regulating PI3K/AKT signal pathway.J Cell Biochem. 2019 Apr;120(4):6781-6788. doi: 10.1002/jcb.27980. Epub 2018 Nov 1. J Cell Biochem. 2019. PMID: 30382631
Cited by
-
SP1-induced lncRNA AGAP2-AS1 expression promotes chemoresistance of breast cancer by epigenetic regulation of MyD88.J Exp Clin Cancer Res. 2018 Aug 29;37(1):202. doi: 10.1186/s13046-018-0875-3. J Exp Clin Cancer Res. 2018. Retraction in: J Exp Clin Cancer Res. 2023 Jan 11;42(1):16. doi: 10.1186/s13046-023-02596-2. PMID: 30157918 Free PMC article. Retracted.
-
Role of MicroRNAs Induced by Chinese Herbal Medicines Against Hepatocellular Carcinoma: A Brief Review.Integr Cancer Ther. 2018 Dec;17(4):1059-1067. doi: 10.1177/1534735418805564. Epub 2018 Oct 20. Integr Cancer Ther. 2018. PMID: 30343602 Free PMC article. Review.
-
Knockdown of lncRNA RMST protect against myocardial infarction through regulating miR-5692 and MAGI3 axis.Am J Transl Res. 2021 Apr 15;13(4):3906-3916. eCollection 2021. Am J Transl Res. 2021. PMID: 34017581 Free PMC article.
-
The Crosstalk Between Regulatory Non-Coding RNAs and Nuclear Factor Kappa B in Hepatocellular Carcinoma.Front Oncol. 2021 Nov 5;11:775250. doi: 10.3389/fonc.2021.775250. eCollection 2021. Front Oncol. 2021. PMID: 34804980 Free PMC article. Review.
-
LncRNA CDKN2BAS predicts poor prognosis in patients with hepatocellular carcinoma and promotes metastasis via the miR-153-5p/ARHGAP18 signaling axis.Aging (Albany NY). 2018 Nov 29;10(11):3371-3381. doi: 10.18632/aging.101645. Aging (Albany NY). 2018. PMID: 30510148 Free PMC article.
References
-
- Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int 2016; 36: 317–324. - PubMed
-
- Razumilava N, Gores GJ. Sorafenib for HCC: a pragmatic perspective. Oncology 2011; 25: 300, 302. - PubMed
-
- Cai L, Wang GJ, Xu ZL, Deng DX, Chakrabarti S, Cherian MG. Metallothionein and apoptosis in primary human hepatocellular carcinoma (HCC) from northern China. Anticancer Res 1998; 18: 4667–4672. - PubMed
-
- Cao W, Li J, Hu C, Shen J, Liu X, Xu Y et al. Symptom clusters and symptom interference of HCC patients undergoing TACE: a cross-sectional study in China. Support Care Cancer 2013; 21: 475–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

