The level of caveolin-1 expression determines response to TGF-β as a tumour suppressor in hepatocellular carcinoma cells
- PMID: 29022911
- PMCID: PMC5680590
- DOI: 10.1038/cddis.2017.469
The level of caveolin-1 expression determines response to TGF-β as a tumour suppressor in hepatocellular carcinoma cells
Abstract
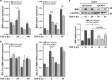
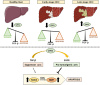
Hepatocellular carcinoma (HCC) is a heterogeneous tumour associated with poor prognostic outcome. Caveolin-1 (CAV1), a membrane protein involved in the formation of caveolae, is frequently overexpressed in HCC. Transforming growth factor-beta (TGF-β) is a pleiotropic cytokine having a dual role in hepatocarcinogenesis: inducer of apoptosis at early phases, but pro-tumourigenic once cells acquire mechanisms to overcome its suppressor effects. Apoptosis induced by TGF-β is mediated by upregulation of the NADPH oxidase NOX4, but counteracted by transactivation of the epidermal growth factor receptor (EGFR) pathway. Previous data suggested that CAV1 is required for the anti-apoptotic signals triggered by TGF-β in hepatocytes. Whether this mechanism is relevant in hepatocarcinogenesis has not been explored yet. Here we analysed the TGF-β response in HCC cell lines that express different levels of CAV1. Accordingly, stable CAV1 knockdown or overexpressing cell lines were generated. We demonstrate that CAV1 is protecting HCC cells from TGF-β-induced apoptosis, which attenuates its suppressive effect on clonogenic growth and increases its effects on cell migration. Downregulation of CAV1 in HLE cells promotes TGF-β-mediated induction of the pro-apoptotic BMF, which correlates with upregulation of NOX4, whereas CAV1 overexpression in Huh7 cells shows the opposite effect. CAV1 silenced HLE cells show attenuation in TGF-β-induced EGFR transactivation and activation of the PI3K/AKT pathway. On the contrary, Huh7 cells, which do not respond to TGF-β activating the EGFR pathway, acquire the capacity to do so when CAV1 is overexpressed. Analyses in samples from HCC patients revealed that tumour tissues presented higher expression levels of CAV1 compared with surrounding non-tumoural areas. Furthermore, a significant positive correlation among the expression of CAV1 and TGFB1 was observed. We conclude that CAV1 has an essential role in switching the response to TGF-β from cytostatic to tumourigenic, which could have clinical meaning in patient stratification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Caveolin-1 is required for TGF-β-induced transactivation of the EGF receptor pathway in hepatocytes through the activation of the metalloprotease TACE/ADAM17.Cell Death Dis. 2014 Jul 17;5(7):e1326. doi: 10.1038/cddis.2014.294. Cell Death Dis. 2014. PMID: 25032849 Free PMC article.
-
Caveolin-1-dependent activation of the metalloprotease TACE/ADAM17 by TGF-β in hepatocytes requires activation of Src and the NADPH oxidase NOX1.FEBS J. 2016 Apr;283(7):1300-10. doi: 10.1111/febs.13669. Epub 2016 Feb 22. FEBS J. 2016. PMID: 26815118
-
Dissecting the effect of targeting the epidermal growth factor receptor on TGF-β-induced-apoptosis in human hepatocellular carcinoma cells.J Hepatol. 2011 Aug;55(2):351-8. doi: 10.1016/j.jhep.2010.10.041. Epub 2010 Dec 13. J Hepatol. 2011. PMID: 21147185
-
Molecular pathogenesis of hepatocellular carcinoma: altering transforming growth factor-β signaling in hepatocarcinogenesis.Dig Dis. 2011;29(3):284-8. doi: 10.1159/000327560. Epub 2011 Aug 9. Dig Dis. 2011. PMID: 21829019 Review.
-
TGF-β signaling in onset and progression of hepatocellular carcinoma.Dig Dis. 2012;30(5):514-23. doi: 10.1159/000341704. Epub 2012 Oct 24. Dig Dis. 2012. PMID: 23108308 Review.
Cited by
-
New and Old Key Players in Liver Cancer.Int J Mol Sci. 2023 Dec 5;24(24):17152. doi: 10.3390/ijms242417152. Int J Mol Sci. 2023. PMID: 38138981 Free PMC article. Review.
-
Caveolin-Mediated Internalization of Fmoc-FF Nanogels in Breast Cancer Cell Lines.Pharmaceutics. 2023 Mar 22;15(3):1026. doi: 10.3390/pharmaceutics15031026. Pharmaceutics. 2023. PMID: 36986886 Free PMC article.
-
Unraveling the Cave: A Seventy-Year Journey into the Caveolar Network, Cellular Signaling, and Human Disease.Cells. 2023 Nov 22;12(23):2680. doi: 10.3390/cells12232680. Cells. 2023. PMID: 38067108 Free PMC article. Review.
-
Proactive and reactive roles of TGF-β in cancer.Semin Cancer Biol. 2023 Oct;95:120-139. doi: 10.1016/j.semcancer.2023.08.002. Epub 2023 Aug 11. Semin Cancer Biol. 2023. PMID: 37572731 Free PMC article. Review.
-
Inhibition of the Caveolin-1 pathway promotes apoptosis and overcomes pan-tyrosine kinase inhibitor resistance in hepatocellular carcinoma.Cell Death Dis. 2025 Jul 25;16(1):561. doi: 10.1038/s41419-025-07887-4. Cell Death Dis. 2025. PMID: 40715090 Free PMC article.
References
-
- Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer 2003; 3: 571–581. - PubMed
-
- Quest AFG, Lobos-González L, Nuñez S, Sanhueza C, Fernández J-G, Aguirre A et al. The caveolin-1 connection to cell death and survival. Curr Mol Med 2013; 13: 266–281. - PubMed
-
- Zhang Z-B, Cai L, Zheng S-G, Xiong Y, Dong J-H. Overexpression of caveolin-1 in hepatocellular carcinoma with metastasis and worse prognosis: correlation with vascular endothelial growth factor, microvessel density and unpaired artery. Pathol Oncol Res 2009; 15: 495–502. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

