Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor
- PMID: 29023413
- PMCID: PMC6151386
- DOI: 10.3390/molecules22101711
Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor
Abstract
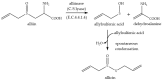
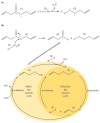
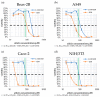
Garlic (Allium sativum) has potent antimicrobial activity due to allicin (diallylthiosulfinate) synthesized by enzyme catalysis in damaged garlic tissues. Allicin gives crushed garlic its characteristic odor and its volatility makes it potentially useful for combating lung infections. Allicin was synthesized (>98% pure) by oxidation of diallyl disulfide by H₂O₂ using formic acid as a catalyst and the growth inhibitory effect of allicin vapor and allicin in solution to clinical isolates of lung pathogenic bacteria from the genera Pseudomonas, Streptococcus, and Staphylococcus, including multi-drug resistant (MDR) strains, was demonstrated. Minimal inhibitory (MIC) and minimal bactericidal concentrations (MBC) were determined and compared to clinical antibiotics using standard European Committee on Antimicrobial Susceptibility Testing (EUCAST) procedures. The cytotoxicity of allicin to human lung and colon epithelial and murine fibroblast cells was tested in vitro and shown to be ameliorated by glutathione (GSH). Similarly, the sensitivity of rat precision-cut lung slices (PCLS) to allicin was decreased by raising the [GSH] to the approximate blood plasma level of 1 mM. Because allicin inhibited bacterial growth as a vapor, it could be used to combat bacterial lung infections via direct inhalation. Since there are no volatile antibiotics available to treat pulmonary infections, allicin, particularly at sublethal doses in combination with oral antibiotics, could make a valuable addition to currently available treatments.
Keywords: Allium sativum; MDR strains; Pseudomonas aeruginosa; Streptococcus pneumoniae; allicin; antimicrobial; garlic; lung pathogenic bacteria; volatile antimicrobial agent.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cavallito C.J., Bailey H.J. Allicin, the antibacterial principle of Allium sativum I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944;66:1950–1951. doi: 10.1021/ja01239a048. - DOI
-
- Cavallito C.J., Buck J.S., Suter C.M. Allicin, the antibacterial principle of Allium sativum II. Determination of the chemical structure. J. Am. Chem. Soc. 1944;66:1952–1954. doi: 10.1021/ja01239a049. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

