Plant Ribosome-Inactivating Proteins: Progesses, Challenges and Biotechnological Applications (and a Few Digressions)
- PMID: 29023422
- PMCID: PMC5666361
- DOI: 10.3390/toxins9100314
Plant Ribosome-Inactivating Proteins: Progesses, Challenges and Biotechnological Applications (and a Few Digressions)
Abstract
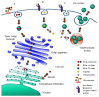
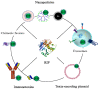
Plant ribosome-inactivating protein (RIP) toxins are EC3.2.2.22 N-glycosidases, found among most plant species encoded as small gene families, distributed in several tissues being endowed with defensive functions against fungal or viral infections. The two main plant RIP classes include type I (monomeric) and type II (dimeric) as the prototype ricin holotoxin from Ricinus communis that is composed of a catalytic active A chain linked via a disulphide bridge to a B-lectin domain that mediates efficient endocytosis in eukaryotic cells. Plant RIPs can recognize a universally conserved stem-loop, known as the α-sarcin/ ricin loop or SRL structure in 23S/25S/28S rRNA. By depurinating a single adenine (A4324 in 28S rat rRNA), they can irreversibly arrest protein translation and trigger cell death in the intoxicated mammalian cell. Besides their useful application as potential weapons against infected/tumor cells, ricin was also used in bio-terroristic attacks and, as such, constitutes a major concern. In this review, we aim to summarize past studies and more recent progresses made studying plant RIPs and discuss successful approaches that might help overcoming some of the bottlenecks encountered during the development of their biomedical applications.
Keywords: ER-stress; nanovectors; plant ribosome inactivating proteins; saporin; targeted drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 1987;262:8128–8130. - PubMed
-
- Endo Y., Mitsui K., Motizuki M., Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 1987;262:5908–5912. - PubMed
-
- Benatti L., Saccardo M.B., Dani M., Nitti G., Sassano M., Lorenzetti R., Lappi D.A., Soria M. Nucleotide sequence of cDNA coding for saporin-6, a type-1 ribosome-inactivating protein from Saponaria officinalis. Eur. J. Biochem. 1989;183:465–470. doi: 10.1111/j.1432-1033.1989.tb14951.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

