The sea cucumber genome provides insights into morphological evolution and visceral regeneration
- PMID: 29023486
- PMCID: PMC5638244
- DOI: 10.1371/journal.pbio.2003790
The sea cucumber genome provides insights into morphological evolution and visceral regeneration
Abstract
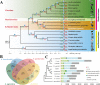
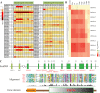
Apart from sharing common ancestry with chordates, sea cucumbers exhibit a unique morphology and exceptional regenerative capacity. Here we present the complete genome sequence of an economically important sea cucumber, A. japonicus, generated using Illumina and PacBio platforms, to achieve an assembly of approximately 805 Mb (contig N50 of 190 Kb and scaffold N50 of 486 Kb), with 30,350 protein-coding genes and high continuity. We used this resource to explore key genetic mechanisms behind the unique biological characters of sea cucumbers. Phylogenetic and comparative genomic analyses revealed the presence of marker genes associated with notochord and gill slits, suggesting that these chordate features were present in ancestral echinoderms. The unique shape and weak mineralization of the sea cucumber adult body were also preliminarily explained by the contraction of biomineralization genes. Genome, transcriptome, and proteome analyses of organ regrowth after induced evisceration provided insight into the molecular underpinnings of visceral regeneration, including a specific tandem-duplicated prostatic secretory protein of 94 amino acids (PSP94)-like gene family and a significantly expanded fibrinogen-related protein (FREP) gene family. This high-quality genome resource will provide a useful framework for future research into biological processes and evolution in deuterostomes, including remarkable regenerative abilities that could have medical applications. Moreover, the multiomics data will be of prime value for commercial sea cucumber breeding programs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, Angerer RC, et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314: 941–952. doi: 10.1126/science.1133609 - DOI - PMC - PubMed
-
- Hall MR, Kocot KM, Baughman KW, Fernandez-Valverde SL, Gauthier MEA, Hatleberg WL, et al. The crown-of-thorns starfish genome as a guide for biocontrol of this coral reef pest. Nature. 2017;544(7649): 231–234. doi: 10.1038/nature22033 - DOI - PubMed
-
- Rychel AL, Swalla BJ. Regeneration in hemichordates and echinoderms. Stem Cells in Mar Organ. 2009: 245–265.
-
- Carnevali MC. Regeneration in Echinoderms: repair, regrowth, cloning. ISJ-Invertebr Surviv J. 2006;3: 64–76.
-
- Mashanov VS, García-Arrarás JE. Gut regeneration in holothurians: a snapshot of recent developments. Biol Bull. 2011;221: 93–109. doi: 10.1086/BBLv221n1p93 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

