Pooled analysis of menstrual irregularities from three major clinical studies evaluating everolimus for the treatment of tuberous sclerosis complex
- PMID: 29023494
- PMCID: PMC5638404
- DOI: 10.1371/journal.pone.0186235
Pooled analysis of menstrual irregularities from three major clinical studies evaluating everolimus for the treatment of tuberous sclerosis complex
Abstract
Objectives: To determine the impact of everolimus on female fertility, including menstrual irregularities, secondary amenorrhea, and luteinizing and follicle stimulating hormone levels in female patients.
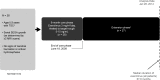
Design: A pooled analysis from 3 prospective studies consisting of a core phase (≥6 months) and a long-term follow-up open-label extension.
Setting: One phase 2 single-center and two phase 3 multicenter studies.
Participants: Data were obtained from female participants, restricted to those between 10 and 55 years of age, during 1 of 3 of the described clinical trials of everolimus. Patients had received ≥ 1 dose of everolimus.
Main outcome measures: Incidence of fertility events.
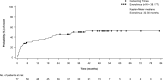
Results: A total of 43/112 patients (38.4%) experienced at least 1 menstrual irregularity. The most common events were amenorrhea (24.1%) and irregular menstruation (17.0%). Seven patients (6.3%) experienced grade 3/4 amenorrhea. When only the longest duration period of amenorrhea for each patient was considered, the median duration was 291 days. Fifteen patients attained menarche during the treatment period in any of the pooled studies. The mean age of menarche for this group was 12.4 years, similar to that of patients who were postmenarche at study entry (12.2 years). A total of 19/92 patients (20.7%) who were postmenarche at baseline or during the study experienced an irregular menstruation event. An increased luteinizing hormone level was reported as an adverse event in 3/112 patients (3%), and follicle-stimulating hormone levels were within normal limits for these patients.
Conclusions: No new safety concerns emerged regarding endocrine function and menstruation in female patients with tuberous sclerosis complex-associated subependymal giant cell astrocytoma or angiomyolipoma, who were receiving everolimus.
Trial registration: ClinicalTrials.gov NCT00411619, NCT00789828, NCT00790400.
Conflict of interest statement
Figures


References
-
- Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615: 125–127. - PubMed
-
- Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuper MA, et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol. 2014;15: 1513–1520. doi: 10.1016/S1470-2045(14)70489-9 - DOI - PubMed
-
- Astrinidis A, Henske EP. Tuberous sclerosis complex: linking growth and energy signaling pathways with human disease. Oncogene. 2005;24: 7475–7481. doi: 10.1038/sj.onc.1209090 - DOI - PubMed
-
- Chromosome European 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75: 1305–1315. - PubMed
-
- van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277: 805–808. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

