Adenovirus vector-based multi-epitope vaccine provides partial protection against H5, H7, and H9 avian influenza viruses
- PMID: 29023601
- PMCID: PMC5638338
- DOI: 10.1371/journal.pone.0186244
Adenovirus vector-based multi-epitope vaccine provides partial protection against H5, H7, and H9 avian influenza viruses
Abstract
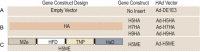
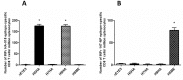
The emergence of H5, H7, and H9 avian influenza virus subtypes in humans reveals their pandemic potential. Although human-to-human transmission has been limited, the genetic reassortment of the avian and human/porcine influenza viruses or mutations in some of the genes resulting in virus replication in the upper respiratory tract of humans could generate novel pandemic influenza viruses. Current vaccines do not provide cross protection against antigenically distinct strains of the H5, H7, and H9 influenza viruses. Therefore, newer vaccine approaches are needed to overcome these potential threats. We developed an egg-independent, adenovirus vector-based, multi-epitope (ME) vaccine approach using the relatively conserved immunogenic domains of the H5N1 influenza virus [M2 ectodomain (M2e), hemagglutinin (HA) fusion domain (HFD), T-cell epitope of nucleoprotein (TNP). and HA α-helix domain (HαD)]. Our ME vaccine induced humoral and cell-mediated immune responses and caused a significant reduction in the viral loads in the lungs of vaccinated mice that were challenged with antigenically distinct H5, H7, or H9 avian influenza viruses. These results suggest that our ME vaccine approach provided broad protection against the avian influenza viruses. Further improvement of this vaccine will lead to a pre-pandemic vaccine that may lower morbidity, hinder transmission, and prevent mortality in a pandemic situation before a strain-matched vaccine becomes available.
Conflict of interest statement
Figures
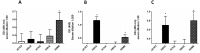
Similar articles
-
Broadly protective adenovirus-based multivalent vaccines against highly pathogenic avian influenza viruses for pandemic preparedness.PLoS One. 2013 Apr 30;8(4):e62496. doi: 10.1371/journal.pone.0062496. Print 2013. PLoS One. 2013. PMID: 23638099 Free PMC article.
-
Vaccine Efficacy of Inactivated, Chimeric Hemagglutinin H9/H5N2 Avian Influenza Virus and Its Suitability for the Marker Vaccine Strategy.J Virol. 2017 Feb 28;91(6):e01693-16. doi: 10.1128/JVI.01693-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077631 Free PMC article.
-
Strategies for inducing protection against avian influenza A virus subtypes with DNA vaccines.Vaccine. 2000 May 22;18(23):2592-9. doi: 10.1016/s0264-410x(99)00485-5. Vaccine. 2000. PMID: 10775793
-
Pandemic preparedness: toward a universal influenza vaccine.Drug News Perspect. 2009 Mar;22(2):80-92. doi: 10.1358/dnp.2009.22.2.1334451. Drug News Perspect. 2009. PMID: 19330167 Review.
-
Progress on adenovirus-vectored universal influenza vaccines.Hum Vaccin Immunother. 2015;11(5):1209-22. doi: 10.1080/21645515.2015.1016674. Hum Vaccin Immunother. 2015. PMID: 25876176 Free PMC article. Review.
Cited by
-
Adenoviral Vector-Based Vaccine Platforms for Developing the Next Generation of Influenza Vaccines.Vaccines (Basel). 2020 Oct 1;8(4):574. doi: 10.3390/vaccines8040574. Vaccines (Basel). 2020. PMID: 33019589 Free PMC article. Review.
-
Immunogenicity and protective efficacy of a multi-antigenic adenovirus-based vaccine candidate against Mycobacterium tuberculosis.Front Microbiol. 2025 Jan 24;16:1492268. doi: 10.3389/fmicb.2025.1492268. eCollection 2025. Front Microbiol. 2025. PMID: 39927262 Free PMC article.
-
A Bovine Adenoviral Vector-Based H5N1 Influenza -Vaccine Provides Enhanced Immunogenicity and Protection at a Significantly Low Dose.Mol Ther Methods Clin Dev. 2018 Jul 17;10:210-222. doi: 10.1016/j.omtm.2018.07.007. eCollection 2018 Sep 21. Mol Ther Methods Clin Dev. 2018. PMID: 30101154 Free PMC article.
-
Construction and Evaluation of the Immunogenicity and Protective Efficacy of Recombinant Replication-Deficient Human Adenovirus-5 Expressing Genotype VII Newcastle Disease Virus F Protein and Infectious Bursal Disease Virus VP2 Protein.Vaccines (Basel). 2023 May 31;11(6):1051. doi: 10.3390/vaccines11061051. Vaccines (Basel). 2023. PMID: 37376440 Free PMC article.
-
Potential cross-species transmission of highly pathogenic avian influenza H5 subtype (HPAI H5) viruses to humans calls for the development of H5-specific and universal influenza vaccines.Cell Discov. 2023 Jun 16;9(1):58. doi: 10.1038/s41421-023-00571-x. Cell Discov. 2023. PMID: 37328456 Free PMC article. Review.
References
-
- Belser JA, Bridges CB, Katz JM, Tumpey TM. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg Infect Dis. 2009;15(6):859–65. Epub 2009/06/16. doi: 10.3201/eid1506.090072 . - DOI - PMC - PubMed
-
- Katz JM, Veguilla V, Belser JA, Maines TR, Van Hoeven N, Pappas C, et al. The public health impact of avian influenza viruses. Poult Sci. 2009;88(4):872–9. Epub 2009/03/12. doi: 10.3382/ps.2008-00465 . - DOI - PubMed
-
- WHO | Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO: World Health Organization; 2017 [updated 2017-08-09 13:07:55]. Available from: http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_tabl....
-
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368(20):1888–97. Epub 2013/04/13. doi: 10.1056/NEJMoa1304459 . - DOI - PubMed
-
- FAO H7N9 situation update—Avian Influenza A(H7N9) virus—FAO Emergency Prevention System for Animal Health (EMPRES-AH) 2017. Available from: http://www.fao.org/ag/againfo/programmes/en/empres/h7n9/situation_update....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

