Guidelines for the use of flow cytometry and cell sorting in immunological studies
- PMID: 29023707
- PMCID: PMC9165548
- DOI: 10.1002/eji.201646632
Guidelines for the use of flow cytometry and cell sorting in immunological studies
Figures
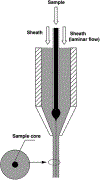


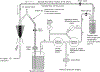
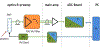
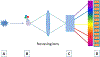














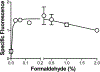
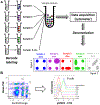





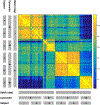





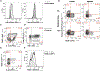




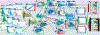

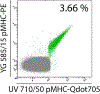


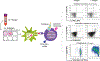




















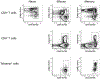
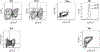





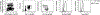
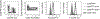



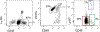

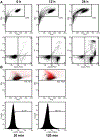
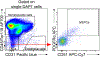


References
-
- Mack J, Fulwyler Particle Separator. US patent US 3380584 A.
-
- Kachel V, Fellner-Feldegg H and Menke E, Hydrodynamic properties of flow cytometry instruments. In Melamed MR, Lindmo T and Mendelsohn ML (Eds.), Flow cytometry and sorting, 2nd ed., Wiley, New York, 1990, pp. 27– 44. ISBN 0-471-56235-1.
-
- Crosland-Taylor PJ, A device for counting small particles suspended in a fluid through a tube. Nature 1953. 171: 37– 38. - PubMed
-
- Gucker FT Jr., O’Konski CT, Pickard HB and Pitts JN Jr., A photoelectronic counter for colloidal particles. J. Am. Chem. Soc 1947. 69: 2422– 2431. - PubMed
-
- Van den Engh GJ, Flow cytometer droplet formation system. US patent US 6861265 B1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

