Oxidative phosphorylation: regulation and role in cellular and tissue metabolism
- PMID: 29023737
- PMCID: PMC5709332
- DOI: 10.1113/JP273839
Oxidative phosphorylation: regulation and role in cellular and tissue metabolism
Abstract
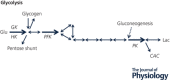
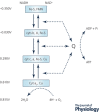
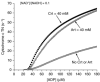
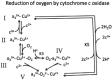
Oxidative phosphorylation provides most of the ATP that higher animals and plants use to support life and is responsible for setting and maintaining metabolic homeostasis. The pathway incorporates three consecutive near equilibrium steps for moving reducing equivalents between the intramitochondrial [NAD+ ]/[NADH] pool to molecular oxygen, with irreversible reduction of oxygen to bound peroxide at cytochrome c oxidase determining the net flux. Net flux (oxygen consumption rate) is determined by demand for ATP, with feedback by the energy state ([ATP]/[ADP][Pi ]) regulating the pathway. This feedback affects the reversible steps equally and independently, resulting in the rate being coupled to ([ATP]/[ADP][Pi ])3 . With increasing energy state, oxygen consumption decreases rapidly until a threshold is reached, above which there is little further decrease. In most cells, [ATP] and [Pi ] are much higher than [ADP] and change in [ADP] is primarily responsible for the change in energy state. As a result, the rate of ATP synthesis, plotted against [ADP], remains low until [ADP] reaches about 30 μm and then increases rapidly with further increase in [ADP]. The dependencies on energy state and [ADP] near the threshold can be fitted by the Hill equation with a Hill coefficients of about -2.6 and 4.2, respectively. The homeostatic set point for metabolism is determined by the threshold, which can be modulated by the PO2 and intramitochondrial [NAD+ ]/[NADH]. The ability of oxidative phosphorylation to precisely set and maintain metabolic homeostasis is consistent with it being permissive of, and essential to, development of higher plants and animals.
Keywords: ATP synthesis; energy metabolism; exercise; metabolic homeostasis; oxidative phosphorylation.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures



References
-
- Buth DG, Murphy RW, Miyamoto MM & Lieb CS (1985). Creatine kinases of amphibians and reptiles: Evolutionary and systematic aspects of gene expression. Copeia 1985, 279–284.
-
- Caster LN & Chance B. (1955) Photochemical action spectra of carbon monoxide‐inhibited respiration. J Biol Chem 217, 453–464. - PubMed
-
- Chacko VP, Aresta F, Chacko SM & Weiss RG (2000). MRI/MRS assessment of in vivo murine cardiac metabolism, morphology, and function at physiological heart rates. Am J Physiol Heart Circ Physiol 279, H2218–H2224. - PubMed
-
- Chance B, Williams GR (1955). Respiratory enzymes in oxidative phosphorylation. I‐IV. J Biol Chem 217, 383–393, 395–407, 409–427, 429–438. - PubMed
-
- Chasiotis D, Sahlin K & Hultman E (1982). Regulation of glycogenolysis in human muscle at rest and during exercise. J Appl Physiol Respir Environ Exerc Physiol 53, 708–715. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

