Dual catenin loss in murine liver causes tight junctional deregulation and progressive intrahepatic cholestasis
- PMID: 29023813
- PMCID: PMC5893443
- DOI: 10.1002/hep.29585
Dual catenin loss in murine liver causes tight junctional deregulation and progressive intrahepatic cholestasis
Abstract
β-Catenin, the downstream effector of the Wnt signaling, plays important roles in hepatic development, regeneration, and tumorigenesis. However, its role at hepatocyte adherens junctions (AJ) is relatively poorly understood, chiefly due to spontaneous compensation by γ-catenin. We simultaneously ablated β- and γ-catenin expression in mouse liver by interbreeding β-catenin-γ-catenin double-floxed mice and Alb-Cre transgenic mice. Double knockout mice show failure to thrive, impaired hepatocyte differentiation, cholemia, ductular reaction, progressive cholestasis, inflammation, fibrosis, and tumorigenesis, which was associated with deregulation of tight junctions (TJ) and bile acid transporters, leading to early morbidity and mortality, a phenotype reminiscent of progressive familial intrahepatic cholestasis (PFIC). To address the mechanism, we specifically and temporally eliminated both catenins from hepatocytes using adeno-associated virus 8 carrying Cre-recombinase under the thyroid-binding globulin promoter (AAV8-TBG-Cre). This led to a time-dependent breach of the blood-biliary barrier associated with sequential disruption of AJ and TJ verified by ultrastructural imaging and intravital microscopy, which revealed unique paracellular leaks around individual hepatocytes, allowing mixing of blood and bile and leakage of blood from one sinusoid to another. Molecular analysis identified sequential losses of E-cadherin, occludin, claudin-3, and claudin-5 due to enhanced proteasomal degradation, and of claudin-2, a β-catenin transcriptional target, which was also validated in vitro.
Conclusion: We report partially redundant function of catenins at AJ in regulating TJ and contributing to the blood-biliary barrier. Furthermore, concomitant hepatic loss of β- and γ-catenin disrupts structural and functional integrity of AJ and TJ via transcriptional and posttranslational mechanisms. Mice with dual catenin loss develop progressive intrahepatic cholestasis, providing a unique model to study diseases such as PFIC. (Hepatology 2018;67:2320-2337).
© 2017 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures
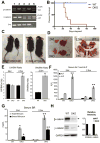
PCR shows various genotypes to identify β-cateninloxp/loxpγ-cateninloxp/loxpAlb-Cre+/− (DKO;2), β-cateninloxp/loxpγ-cateninloxp/WTAlb-Cre+/− (4), GKO (β-cateninloxp/WTγ-cateninloxp/loxpAlb-Cre+/− (1), β-cateninloxp/WTγ-cateninloxp/WTAlb-Cre+/− (3), and β-cateninloxp/loxpγ-cateninloxp/loxpAlb-Cre-/- (WT controls; 5)
Kaplan-Meier curve shows significant mortality in DKO mice.
Both male (M) and female (F) DKO mice show stunted growth compared to WT.
DKO livers at 24d and 2.5m exhibit yellow color and stiffness; and large spleens (2.5m).
Liver weight/body weight ratio (LW/BW) remains unchanged in 24d DKO. At 2.5m, DKO show increased LW/BW as compared to WT (p=0.0056). Spleen weight/body weight ratio (SW/BW) is also significantly increased in DKO (p=0.0022).
High serum ALP in DKO mice (p=0.0004 at 24d, p=0.0039 at 2.5m) compared to WT. No significant changes in ALT levels.
Significantly higher total (p=0.0007 at 24 days and p= 0.0008 at 2.5 months) and direct (p=0.0026 at 24 days and p= 0.0006 at 2.5 months) serum BR levels in DKO than WT.
Representative WB of β- and γ-catenin proteins in WT and DKO livers. GAPDH shown as loading control. Densitometry verified around 8-fold reduction in β- and γ-catenin in DKO liver.
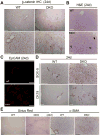
Liver IHC shows membranous staining of β-catenin in WT, which is lost in DKO.
H&E shows notable increase in periportal progenitors at 24d in DKO livers.
IF for EpCAM shows increased ductular cells in DKO.
IHC for Sox-9 shows localization to bile ducts in WT. In DKO, Sox-9 also localizes to periportal hepatocytes. Increased CK-19 positive ductular reaction also seen in DKO.
Sirius red staining shows increased collagen in DKO livers. Increased α-SMA-positive active-myofibroblasts also observed in DKO livers.
Scale bars: 50μm.
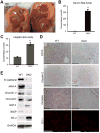
Gall bladder (arrow) are involuted and lack bile in DKO but not in WT mice.
A significant increase in serum BA in DKO as compared to WT (p=0.0001).
A significant increase in hepatic BA in DKO as compared to WT (p=0.0002).
IHC on liver sections show loss of CD10, claudin-2 and occludin, and IF shows decreased ZO-1 in DKO liver. Scale bar: 50μm.
WB shows decreased protein levels of E-cadherin, JAM-A, claudin-2, occludin and BSEP and increase in GGT and ZO-2 in DKO as compared to WT liver lysates. GAPDH was loading control.
Intravital image of dWT liver shows FITC dextran labeled liver sinusoids; DKO2 liver shows presence of thin conduits around hepatocytes (*) that connect one sinusoid to another. Scale bars: 10μm.
Time series images (5m, 10m and 30m) of (n=1) 12d dWT liver shows localization of CF (green) and Texas-red dextran (red) in bile canaliculi and sinusoids, respectively. Time series images of 12d DKO2 liver shows co-localization of CF and Texas-red dextran in sinusoids and failure of uptake and secretion into bile canaliculi at 5m, 10m and 30m after injection of dyes. Instead, there was appearance of thin conduits or collaterals between hepatocytes through which red and green positive aggregates move and are also seen accumulating in peri-sinusoidal areas (asterisk). Scale bars: 50μm.
Quantification of Evans blue in the blood and bile of 10d dWT and DKO2 mice sampled 15 minutes after Evans blue injection into the inferior vena cava shows no appearance of dye in bile. At 12d, significant increase in Evans blue dye is observed in DKO2 bile as compared to dWT (p=0.001).
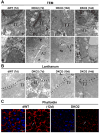
TEM of 7d dWT and DKO2 livers showed presence of electron-dense and normal appearing TJ close to biliary canaliculi (BC). Normal appearing AJ seen at 7d in dWT, while increased intercellular space (left-right arrow) evident in DKO2 livers at this time-point. TJ are less electron-dense at 10d in DKO2 while AJ continue to show loose structure. Widened TJ and AJ (left-right arrow) evident in DKO2 liver sections at 14d.
D7 dWT and DKO2 liver sections showed intact TJ devoid of lanthanum hydroxide (black particles) while AJ are positive (arrows). DKO2 liver sections at 12d and 14d show lanthanum hydroxide inside the TJ (arrows).
Phalloidin staining in DKO2 liver at 12d shows lack of classical train-track pattern of localization outlining the biliary canaliculi as seen in dWT liver (arrow) at the same time. Arrow in DKO2 points to decreased organization, collapse and incomplete of network. Scale bars: 50μm.
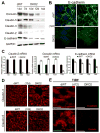
Representative WB shows decreased E-cadherin at 10d-14d, and occludin, claudin-3 and -5 at 12d-14d in DKO2 as compared to 14d dWT. Claudin-2 was absent from 7d in DKO2 livers. GAPDH shows protein loading.
RT-PCR shows significant decrease in expression of claudin-2 (p<0.001) in DKO2 at 7d onwards as compared to dWT livers. Significant decrease in occludin and E-cadherin mRNA in DKO2 livers was evident only at 14d (p<0.001).
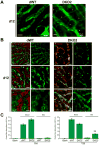
Membrane localization of E-cadherin evident in dWT livers was visibly reduced in DKO2 at 12d and appeared as punctate cytoplasmic staining by confocal microscopy.
Confocal microscopy showed membrane localization of claudin-3 and -5 in dWT livers, which was reduced in DKO2 at 12d.
TIRF imaging revealed membrane localization of E-cadherin in dWT liver sections, which was absent in DKO2 livers at 12d.
TIRF imaging revealed membrane localization of occludin in dWT liver sections, which was absent in DKO2 livers at 12d. G. Scale bars: 50μm.

Immunoprecipitation (IP) of occludin from lysates of Hep3B cells transfected with scrambled (Sc) or dual siRNA against β- and γ-catenin (SiBG) showed its association with E-cadherin, β-catenin and γ-catenin in Sc only. IP of γ-catenin in these cells showed reduced association of γ-catenin with occludin in Hep3B cells transfected with siRNA against γ-catenin (SiG) and SiBG but not Sc or siRNA against β-catenin (SiB).
MG132 (MG) and cyclohexamide (C) treatment of Sc or SiBG Hep3B cells showed MG treatment alone stabilized E-cadherin and occludin in SiBG.
SiBG-transfected Hep3B cells showed decreased protein levels of occludin, claudin-3 and claudin-2. SiB-transfection alone led to decreased claudin-2 as well. MG treatment of SiBG-transfected cells rescued occludin and claudin-3 but not claudin-2 levels.
Comparable E-cadherin pull-down associated with more ubiquitin by IP in SiBG-transfected Hep3B cell lysates as compare to Sc. Densitometry showed ∼1.7-fold increase in ubiquitination.
SiRNA against E-cadherin (SiE)-transfected Hep3B cells showed decreased protein levels of occludin and claudin-3 but no change in β- or γ-catenin, GAPDH verified protein loading.
Comparable E-cadherin pull-down associated with more ubiquitin by IP especially at 14d in DKO2 liver lysates. Densitometry verifies around 5-fold increase in E-cadherin ubiquitination in DKO2 livers at 14d.
IP of occludin followed by WB with ubiquitin showed increased ubiquitination of occludin in DKO2 liver samples at 7d and 14d. Densitometry showed around 2-fold and 9-fold increase in occludin ubiquitination at 7d and 14d, respectively.
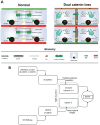
TJ, AJ, and desmosomes exist between two adjacent hepatocytes. TJ prevent mixing of blood (red) in sinusoid and bile (green) in canaliculi, and prevent mixing of blood from adjacent sinusoids. Dual loss of catenins at AJ affects TJ to disrupt hepatic tissue barrier, resulting in para-hepatocyte leaks allowing mixing of blood and bile, and blood from one sinusoid to another.
A model depicting distinct mechanisms by which β-catenin regulates hepatic TJ integrity. At AJ, β-catenin or in its absence γ-catenin, stabilizes E-cadherin and in turn stabilizes TJ proteins occludin and plaque-forming claudins -3 and -5. As a component of the Wnt signaling pathway, β-catenin non-redundantly regulates expression of claudin-2 essential for biliary canalicular structure and bile flow. Loss of β-catenin alone affects claudin-2 expression only, but dual loss of catenins additionally affects occludin and plaque-forming claudins to affect TJ structure and barrier function.
Comment in
-
Dual ablation of β- and γ-catenin: Critical regulators of junctions and their functions.Hepatology. 2018 Jun;67(6):2079-2081. doi: 10.1002/hep.29761. Epub 2018 Apr 19. Hepatology. 2018. PMID: 29272046 Free PMC article.
References
-
- Brembeck FH, Rosário M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Current opinion in genetics & development. 2006;16:51–59. - PubMed
-
- Bienz M. beta-Catenin: a pivot between cell adhesion and Wnt signalling. Current biology : CB. 2005;15:R64–67. - PubMed
-
- Behari J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, Apte U, et al. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. The American journal of pathology. 2010;176:744–753. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

