Myeloid Notch1 deficiency activates the RhoA/ROCK pathway and aggravates hepatocellular damage in mouse ischemic livers
- PMID: 29024000
- PMCID: PMC5826840
- DOI: 10.1002/hep.29593
Myeloid Notch1 deficiency activates the RhoA/ROCK pathway and aggravates hepatocellular damage in mouse ischemic livers
Abstract
Notch signaling plays an emerging role in the regulation of immune cell development and function during inflammatory response. Activation of the ras homolog gene family member A/Rho-associated protein kinase (ROCK) pathway promotes leukocyte accumulation in tissue injury. However, it remains unknown whether Notch signaling regulates ras homolog gene family member A/ROCK-mediated immune responses in liver ischemia and reperfusion (IR) injury. This study investigated intracellular signaling pathways regulated by Notch receptors in the IR-stressed liver and in vitro. In a mouse model of IR-induced liver inflammatory injury, we found that mice with myeloid-specific Notch1 knockout showed aggravated hepatocellular damage, with increased serum alanine aminotransferase levels, hepatocellular apoptosis, macrophage/neutrophil trafficking, and proinflammatory mediators compared to Notch1-proficient controls. Unlike in the controls, myeloid Notch1 ablation diminished hairy and enhancer of split-1 (Hes1) and augmented c-Jun N-terminal kinase (JNK)/stress-activated protein kinase-associated protein 1 (JSAP1), JNK, ROCK1, and phosphatase and tensin homolog (PTEN) activation in ischemic livers. Disruption of JSAP1 in myeloid-specific Notch1 knockout livers improved hepatocellular function and reduced JNK, ROCK1, PTEN, and toll-like receptor 4 activation. Moreover, ROCK1 knockdown inhibited PTEN and promoted Akt, leading to depressed toll-like receptor 4. In parallel in vitro studies, transfection of lentivirus-expressing Notch1 intracellular domain promoted Hes1 and inhibited JSAP1 in lipopolysaccharide-stimulated bone marrow-derived macrophages. Hes1 deletion enhanced JSAP1/JNK activation, whereas clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9-mediated JSAP1 knockout diminished ROCK1/PTEN and toll-like receptor 4 signaling.
Conclusion: Myeloid Notch1 deficiency activates the ras homolog gene family member A/ROCK pathway and exacerbates hepatocellular injury by inhibiting transcriptional repressor Hes1 and inducing scaffold protein JSAP1 in IR-triggered liver inflammation; our findings underscore the crucial role of the Notch-Hes1 axis as a novel regulator of innate immunity-mediated inflammation and imply the therapeutic potential for the management of organ IR injury in transplant recipients. (Hepatology 2018;67:1041-1055).
© 2017 by the American Association for the Study of Liver Diseases.
Figures


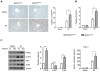
 ) and Notch1M-KO (
) and Notch1M-KO (
 ) mice. (A) Immunofluorescence staining of CD11b+ macrophages in ischemic livers. Quantification of CD11b+ macrophages per high power field. Results scored semi-quantitatively by averaging number of positively-stained cells (mean±SD)/field at 200×magnification. Representative of 4-6 mice/group. *p<0.05. (B) Quantitative RT-PCR-assisted detection of TNF-α, IL-1β, and MCP-1 in mouse livers. Each column represents the mean±SD (n=3-4 samples/group). *p<0.05, **p<0.01. (C) Immunohistochemistry staining of Ly6G+ neutrophils in ischemic livers. Quantification of Ly6G+ neutrophils per high power field (original magnification ×200). Representative of 4-6 mice/group. **p<0.01.
) mice. (A) Immunofluorescence staining of CD11b+ macrophages in ischemic livers. Quantification of CD11b+ macrophages per high power field. Results scored semi-quantitatively by averaging number of positively-stained cells (mean±SD)/field at 200×magnification. Representative of 4-6 mice/group. *p<0.05. (B) Quantitative RT-PCR-assisted detection of TNF-α, IL-1β, and MCP-1 in mouse livers. Each column represents the mean±SD (n=3-4 samples/group). *p<0.05, **p<0.01. (C) Immunohistochemistry staining of Ly6G+ neutrophils in ischemic livers. Quantification of Ly6G+ neutrophils per high power field (original magnification ×200). Representative of 4-6 mice/group. **p<0.01.

 ) or ROCK1 siRNA (
) or ROCK1 siRNA (
 ) (2 mg/kg) mixed with mannose-conjugated polymers at 4h prior to ischemia. (A) The severity of liver IRI was evaluated by the Suzuki's histological grading at 6h of reperfusion followed by 90min of ischemia. *p<0.05. (B) Hepatocellular function was evaluated by sALT levels (IU/L). Results expressed as mean±SD (n=4-6 samples/group). **p<0.01. (C) Immunofluorescence staining of CD11b+ macrophages in ischemic livers. Quantification of CD11b+ macrophages per high power field. Results scored semi-quantitatively by averaging number of positively-stained cells (mean±SD)/field at 200×magnification. Representative of 4-6 mice/group. **p<0.01. (D) Immunohistochemistry staining of Ly6G+ neutrophils in ischemic livers. Quantification of Ly6G+ neutrophils per high power field (original magnification ×200). Representative of 4-6 mice/group. *p<0.05. (E) Western blots analysis and relative density ratio of PTEN, p-Akt, and TLR4. Representative of three experiments. *p<0.05, **p<0.01. (F) Quantitative RT-PCR-assisted detection of mRNA coding for TNF-α, IL-1β, and MCP-1. Each column represents the mean±SD (n=3-4 samples/group). *p<0.05, **p<0.01.
) (2 mg/kg) mixed with mannose-conjugated polymers at 4h prior to ischemia. (A) The severity of liver IRI was evaluated by the Suzuki's histological grading at 6h of reperfusion followed by 90min of ischemia. *p<0.05. (B) Hepatocellular function was evaluated by sALT levels (IU/L). Results expressed as mean±SD (n=4-6 samples/group). **p<0.01. (C) Immunofluorescence staining of CD11b+ macrophages in ischemic livers. Quantification of CD11b+ macrophages per high power field. Results scored semi-quantitatively by averaging number of positively-stained cells (mean±SD)/field at 200×magnification. Representative of 4-6 mice/group. **p<0.01. (D) Immunohistochemistry staining of Ly6G+ neutrophils in ischemic livers. Quantification of Ly6G+ neutrophils per high power field (original magnification ×200). Representative of 4-6 mice/group. *p<0.05. (E) Western blots analysis and relative density ratio of PTEN, p-Akt, and TLR4. Representative of three experiments. *p<0.05, **p<0.01. (F) Quantitative RT-PCR-assisted detection of mRNA coding for TNF-α, IL-1β, and MCP-1. Each column represents the mean±SD (n=3-4 samples/group). *p<0.05, **p<0.01.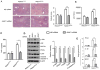
 ) or JSAP1 siRNA (
) or JSAP1 siRNA (
 ) (2 mg/kg) mixed with mannose-conjugated polymers at 4h prior to ischemia. (A) Representative histological staining (H&E) of ischemic liver tissue at 6h of reperfusion followed by 90min of ischemia. Results representative of 4-6 mice/group; original magnification ×100. The severity of liver IRI was evaluated by the Suzuki's histological grading. **p<0.01. (B) Hepatocellular function was evaluated by sALT levels (IU/L). Results expressed as mean±SD (n=4-6 samples/group). **p<0.01. (C) ELISA analysis of TNF-α levels in animal serum. Mean±SD (n=3-4 samples/group), **p<0.01. (D) Western blots analysis and relative density ratio of p-JNK, ROCK1, PTEN, TLR4, and cleaved caspase-3. Representative of three experiments. *p<0.05, **p<0.01. (E) Quantitative RT-PCR-assisted detection of mRNA coding for RhoA, IL-1β, and MCP-1. Each column represents the mean±SD (n=3-4 samples/group). **p<0.01.
) (2 mg/kg) mixed with mannose-conjugated polymers at 4h prior to ischemia. (A) Representative histological staining (H&E) of ischemic liver tissue at 6h of reperfusion followed by 90min of ischemia. Results representative of 4-6 mice/group; original magnification ×100. The severity of liver IRI was evaluated by the Suzuki's histological grading. **p<0.01. (B) Hepatocellular function was evaluated by sALT levels (IU/L). Results expressed as mean±SD (n=4-6 samples/group). **p<0.01. (C) ELISA analysis of TNF-α levels in animal serum. Mean±SD (n=3-4 samples/group), **p<0.01. (D) Western blots analysis and relative density ratio of p-JNK, ROCK1, PTEN, TLR4, and cleaved caspase-3. Representative of three experiments. *p<0.05, **p<0.01. (E) Quantitative RT-PCR-assisted detection of mRNA coding for RhoA, IL-1β, and MCP-1. Each column represents the mean±SD (n=3-4 samples/group). **p<0.01.
 ) Cells only; (
) Cells only; (
 ) LV-pSin-NICD; (
) LV-pSin-NICD; (
 ) LPS + LV-Hes1 KO; (
) LPS + LV-Hes1 KO; (
 ) LPS + LV-control; (
) LPS + LV-control; (
 ) LPS + LV-JSAP1 KO.
) LPS + LV-JSAP1 KO.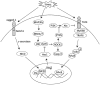
References
-
- Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. - PubMed
-
- Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. - PubMed
-
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

